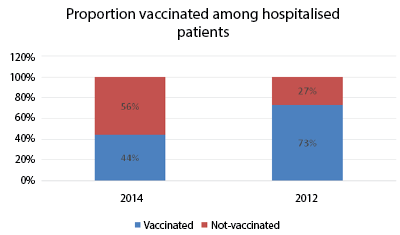
Figure 1: Proportion of vaccinated versus non-vaccinated patients admitted with confirmed influenza A(H1N1); 2014, n=100; 2012, n=92
Louis Han1 Ashley Ha2 Lance C Jennings3 Lutz Beckert4*
1Medical student, University of Otago Christchurch, New Zealand*Corresponding author: Prof Lutz Beckert, Department of Respiratory Medicine, 1 Riccarton Avenue, PO Box 4345, Christchurch 8140, New Zealand, Tel: +6433640640; Fax: 03 3640914; E-mail: Lutz.Beckert@cdhb.health.nz
Aims: To compare the clinical outcomes in patients who were admitted to Christchurch Hospital with laboratory confirmed influenza A(H1N1) pdm09 and who had received the 2014 trivalent influenza vaccine with those who had not received the vaccine, then to relate the pattern to similar patients admitted in 2012.
Methods: 100 consecutive hospital in-patients with laboratory confirmed influenza A(H1N1)pdm09 virus infection in 2014 were identified from the laboratory information system. Demographic information was obtained; all patients were contacted and asked about their vaccination status prior to hospital admission. Above process was done for those with laboratory confirmed influenza A(H3N2) in 2012. The 2014 results were compared to the observations from 2012, an A(H3N2) virus dominant year.
Results: In 2012, 92 patients with laboratory confirmed influenza responded and 67 reported receiving the 2012 trivalent influenza vaccine. In 2014, 44 of 100 patients reported receiving the trivalent influenza vaccine at least two weeks prior to their hospital admission. There were a significantly lower proportion of vaccinated patients admitted in 2014 compared to 2012 (44% vs 73%), mean age was younger (48.5 vs 80 years). There was no significant difference between the cohorts in final outcomes: length of stay, ICU admissions, and deaths.
Conclusions: We found that fewer patients admitted in 2014 had been vaccinated against influenza and that the average age was lower than those patients admitted in 2012. It is possible that the clinical effectiveness, especially in the elderly was greater, with the 2014 vaccine.
Influenza A virus; Trivalent influenza vaccine
Influenza is a common respiratory disease caused by influenza type A and B viruses, which can result in serious outcomes including pneumonia, hospital admission and death. Influenza vaccination provides the best protection against influenza and is widely recommended [1]. Each year the World Health Organization (WHO) makes recommendations on the influenza vaccine composition for the upcoming Northern and Southern hemisphere seasonal influenza vaccines. In 2012, a Christchurch New Zealand study and the Southern Hemisphere Influenza and Vaccine Effectiveness, Research and Surveillance (SHIVERS) project reported the apparent lower effectiveness of the 2012 trivalent vaccine, particular in the elderly population [2,3]. The Christchurch study was based on hospitalized patients with confirmed influenza, and the SHIVERS study was a case – test negative study on those admitted with severe acute respiratory infection (SARI) to Auckland hospitals. Estimated vaccine overall effectiveness was 39% but it was only 8% for patients aged 65 years and over. This was subsequently partially explained by an antigenic drift observed in circulating strain compared to the A(H3N2) component of the trivalent vaccine [4].
In 2014, the Southern Hemisphere influenza vaccine composition included an A/California/7/2009 (H1N1)-like virus, A/Texas/50/2012 (H3N2)-like virus and B/Massachusetts/2/2012-like virus [5,6]. This vaccine was available for use by all New Zealanders, and was free of charge for those at-risk of serious influenza; pregnant woman, people with asthma, diabetes, heart disease, renal disease, cancer or another serious medical condition, those aged ≥65 years as well as children under 5 years with a history of significant respiratory illness [1]. New Zealand’s influenza season in 2014 followed the typical seasonal winter activity, peaking between late July and mid-September [7]. Influenza A(H1N1) pdm09 was the dominant virus.
In 2012, there was mismatch between vaccine composition and circulating strains. Influenza vaccine composition included A/ California/7/2009(H1N1)pdm09-like strain A/Perth/16/2009(H3N2)-like strain, and B/Brisbane/60/2008-like strain [8,9]. However, the dominant H3N2 strain was closely related to A/Victoria/361/2011 (H3N2) [10].
The aim of this study was to explore the clinical outcomes in patients who were admitted to Christchurch Hospital with laboratory confirmed influenza A(H1N1)pdm09 infection. We explored the effectiveness of the 2014 trivalent influenza vaccine and also compared our findings to the 2012 influenza season.
A total of 100 consecutive hospital in-patients with laboratory confirmed influenza A(H1N1) infection were retrospectively selected from the Canterbury Health Laboratory Information System database. Canterbury Health Laboratory processes all samples from Christchurch Hospital. Each patient was contacted by telephone and asked whether they had been vaccinated with the Southern Hemisphere seasonal trivalent influenza vaccine in 2014.
Participants were classified as being vaccinated if they reported having received the influenza vaccine in the current season and had received the vaccine at least 2 weeks before the onset of their illness. Patient demographic information was collected from the clinical repository database and data for both vaccinated and non-vaccinated participants was compared.
Smoking status was based on whether a patient was a current smoker at the time of their admission. Pre-existing lung disease was defined as doctor diagnosed chronic lung disease; diseases included conditions such as COPD, asthma and interstitial lung disease. This audit was approved by the Ethics Committee of the University of Otago (approval number: HD14/37).
2012 data was collected in the same manner, starting from July 2012. Two-way ANOVA was used to compare the mean age and length of admissions for two groups. Fisher’s exact test was used to calculate any differences. A p-value of ≤ 0.05 was considered statistically significant.
For 2014, of 102 consecutive patients contacted, two were not enrolled; one did not consent for interview, while the other had advanced dementia and did not have the capacity to consent. A total of 44 (44%) participants with laboratory confirmed influenza A(H1N1)pdm09 infection had received an influenza vaccination at least two weeks prior to their hospital admission. In comparison, in the 2012 study 67 (73%) patients had been vaccinated. There was statistically very significant difference in the proportion of vaccinated during 2014 and 2012 (p-value 0.001) (Figure 1).

Figure 1: Proportion of vaccinated versus non-vaccinated patients admitted with confirmed influenza A(H1N1); 2014, n=100; 2012, n=92
In year 2014, the vaccinated group was older compared to 2012 (mean age 57.4 vs. 39.7, p-value 0.001), had a lower proportion of smokers (9.1% vs. 37.5%, p-value 0.001) and more patients with lung disease (48% vs 23%, p-value 0.01).
There was no significant difference in mean length of hospital stay (5.55 vs 5.48 days, p-value 0.98) or ICU admission (2.3% vs 5.4%, p-value 0.63) between vaccinated and non-vaccinated patients in 2014. There were no deaths in either group during admission.
The major differences between the 2012 and 2014 studies were that the number of vaccinated patients, who were admitted with confirmed influenza A infection was smaller in 2014 compared to 2012 (44% vs 73%, p-value 0.001) and that their mean age was younger in 2014 compared to 2012 (mean age 48.5 vs 80 years, p-value 0.001) (Tables 1 and 2).
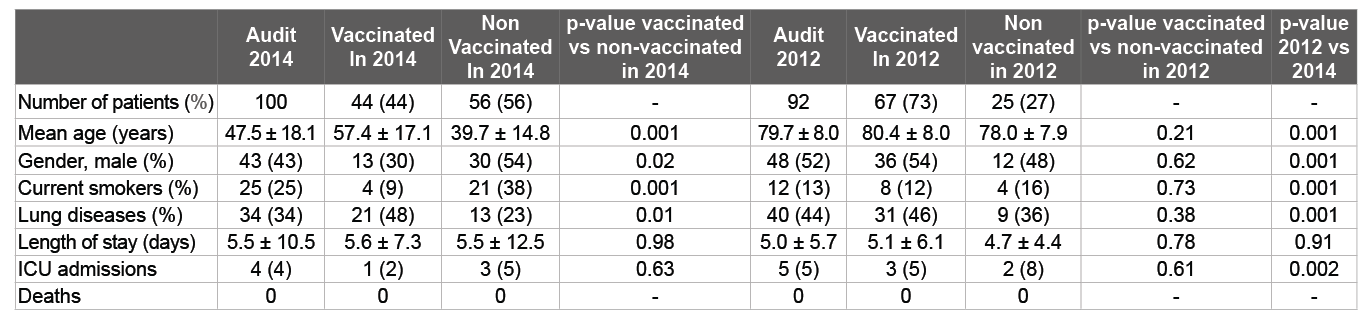
Table 1: Demographic data of the participants who were admitted with laboratory confirmed influenza A) in winter 2014 and 2012 in the Canterbury District Health Board area of New Zealand
Respiratory condition |
Number in 2014 |
Number in 2012 |
COPD |
10 |
27 |
- Severe |
4 |
12 |
- Moderate |
1 |
2 |
- Mild |
0 |
4 |
- No spirometry |
5 |
9 |
Asthma |
18 |
3 |
Nodules |
|
2 |
Lung cancer |
|
1 |
Pneumonitis |
|
1 |
Interstitial lung disease |
|
1 |
Chronic bronchitis |
|
1 |
OSA |
2 |
1 |
Asbestosis |
|
1 |
Childhood TB |
|
1 |
Nitrofurantoin lung |
|
1 |
Sarcoidosis |
|
|
Restrictive lung disease |
1 |
|
Bronchiolitis |
1 |
|
Bronchiectasis |
1 |
|
Muscular dystrophy |
1 |
|
TOTAL |
34 |
40 |
Table 2: Comorbidities of patients admitted with confirmed influenza A(H3N2) in winter 2012
COPD classification based on FEV1 predicted– severe<50%, moderate 50-80%, mild>80.
The two key findings from these audits of patients admitted during the influenza season with laboratory confirmed influenza A infection in 2014 and in 2012 were: first, the percentage of vaccinated patients in 2014 (44%) was considerably lower than in 2012 (73%) and second, the average age of patients admitted in 2014 was younger, suggesting a better protection of one of the vaccination target groups patients 65 years and over of age.
A major difference between these two influenza seasons was that in 2012 an A(H3N2) virus was the dominant circulating virus, while in 2014, an A(H1N1)pdm09 virus was dominant.
The reduced effectiveness of the seasonal influenza vaccine particularly in the elderly population was reported in two studies in New Zealand in 2012 [2,3]. Patients admitted with influenza in 2014 were overall younger than those admitted in 2012, suggesting a possible improvement in the effectiveness of the 2014 trivalent vaccine, although this could just be characteristic of the epidemiology of the dominant viruses with the A(H1N1)pdm09 virus not leading to severe disease outcome in the elderly this season.
The proportion of patients with underlying lung disease both in the 2014 and 2012 was high. This finding is not surprising as we would expect a worse health outcome in the selected group. Patients admitted in both years with underlying lung disease were more likely to have been vaccinated, raising the possibility of lower vaccine effectiveness in these patients, although neither study was designed to investigate this. Those with underlying lung disease may have different health related behavior, leading to increased uptake of vaccination. This would be further complemented by New Zealand Immunization strategy offering funded vaccine. It is important to note that underlying lung disease may be a confounder in this study, as it would impact on the rate of admission from influenza infection, as well as vaccination rate.
The smoking rate of patients hospitalized in 2014 with influenza A(H1N1) pdm09 infection was 25%. It is considerably higher than the smoking rate of the general Canterbury population (14%) [11]. The reason for this is unclear, we speculate that this may reflect smoking being associated with other co morbidities that would predispose to a higher risk of severe influenza and other adverse health outcomes. It was reassuring to see that the number of vaccinated smokers presenting in 2014 with influenza was small despite a high degree of lung disease in this study. Possible explanations for this observation are that more nonsmokers sought vaccination, as they were more conscious of personal health and illness prevention issues. Alternatively, some patients in this group who had chronic lung disease may have stopped smoking because of the adverse health effects.
Although a smaller number of vaccinated individuals were admitted in 2014 compared to 2012, possible reasons are confounded by the different viruses, which circulated in the Canterbury region in 2012 and 2014. Typical seasonal influenza patterns were observed in New Zealand during the winter influenza seasons of 2012 and 2014 with overall activity lower in 2014 [12]. In 2012, there was an antigenic mismatch between seasonal vaccine A(H3N2) component and the circulating A(H3N2) strain in New Zealand, possibly impacting on the higher numbers of vaccinated individuals observed in the 2012 audit.
Neither survey demonstrated a significant difference between clinical outcomes of vaccinated and non-vaccinated patients admitted to hospital with laboratory confirmed influenza A infection. They did show that the number of vaccinated patients admitted with laboratory confirmed influenza A infection was smaller in 2014 compared to 2012.
These studies have several limitations. Firstly, we are unable to comment on hospital admissions avoided. Secondly, the numbers of admitted patients are small, so a possible protective effect on the severity of illness or length of hospital stay may not have been detected. Finally, we can only comment on possible vaccine effectiveness in terms of disease prognosis in hospitalized patients.
Influenza vaccination provides the best protection against influenza. Although the vaccine is less effective at preventing clinical illness in older people, influenza vaccination does reduce hospitalization and deaths. How well the vaccine works as a public health intervention varies from influenza season to season depending on the age of the individual, their health status, their immune status and the closeness of the “match’ of the vaccine composition and the influenza virus strains circulating in the community. Thus the measuring of vaccine outcomes is problematic. The best measure of vaccine outcome is vaccine efficacy gained from randomized controlled trials comparing vaccinated and unvaccinated individuals where infection is laboratory-confirmed. These studies are difficult to conduct, thus vaccine effectiveness is the usual outcome measure which is obtained from less robust observational, un-randomised studies.
The effectiveness of inactivated vaccines against influenza in systematic reviews and meta-analyses has ranged from 59% (95% CI 51-67) [13] to 73% (54-84) [14] in healthy adults for years where the vaccine and circulating strains were well matched. While the re-analysis of the data from the Cochrane Review on effectiveness of influenza vaccines in the elderly from a biological perspective, has shown the inactivated influenza vaccine effectiveness to be 28% (95% CI 26-30) against non-fatal and fatal complications, 39% (35-43) against influenza-like illness and 49% (33-62) for laboratory confirmed influenza in elderly individuals 65 years and over [15].
Observational studies termed “case test-negative” studies where the outcomes among individuals who have been vaccinated and have laboratory confirmed influenza infection are compared with those who have no laboratory confirmed influenza, are now providing more timely assessments of vaccine effectiveness. New Zealand data from the SHIVERS study has shown that the inactivated influenza vaccine used during the current 2014 study was 54% (95% CI 19-74) effective in preventing influenza hospitalisation and 67% (48-79) effective against presentation to a general practice with an influenza-like illness [16].
In summary, both results of the 2012 and 2014 study on the effectiveness of the seasonal influenza vaccine are reassuring. We found that fewer laboratory confirmed influenza patients admitted in 2014 had been vaccinated against influenza and that the average age was lower than those patients admitted in 2012. It is possible that the clinical effectiveness, especially in the elderly was greater with the 2014 vaccine. While these studies have limitations, health practitioners should continue to encourage the uptake of the seasonal influenza vaccine.
Download Provisional PDF Here
Article Type: Research Article
Citation: Han L, Ha A, Jennings LC, Beckert L (2017) Survey of Patients Admitted to Hospital with Laboratory Confirmed Influenza A virus Infection in 2012 and 2014–Lessons for the Future. Int J Vaccine Immunizat 3(1): doi http://dx.doi.org/10.16966/2470-9948.114
Copyright: © 2017 Han L, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Publication history:
All Sci Forschen Journals are Open Access