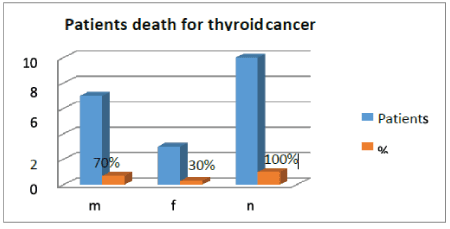
Figure 1: Patients death for thyroid cancer.


Francisco Medrano1* Roger Matus G2 Carmen Quezada3 Guillermo Carmona4
1Head and Neck Surgeon, Oncologic program Salud Integral Hospital, Managua, Nicaragua*Corresponding authors: Francisco Medrano, Head and Neck Surgeon, Oncologic program Salud Integral Hospital, Managua, Nicaragua, Tel: 505-8880-5968; E-mail: fmedranos@hotmail.com
The thyroid cancer is the most indolent as death cause, the good prognosis, generally is the rule on the exception, however, the death by this disease has not change yet. Between 10-15 % of patients with thyroid cancer can present tumors with aggressive behavior, developing locally and regional advanced disease, or metastatic disease. Several research has reported a small number of fatal cases, consequently these has avoided developing guide or protocols to treat the patients. The goal of this study is to know the cause of death in patients with thyroid cancer.
Method: Descriptive study, series of cases in a period of 14 years (January 2003-December 2017). We have reviewed the file records of 250 patients with thyroid cancer, the clinical characteristic were reviewed, we used the histology subtype as WHO (World Health Organization) and AGES classification to determine the risk factor of dying. The univariable review was realized with frequency and percentage. The overall survival was done by using Kaplan-Meier.
Results: 10 dead patients, represent 4%. Male 7(70%), women 3(30%), age range was 54-65 years old, the average age was 59.2 years old. The papillary thyroid cancer was 9 cases, 3 (30%) classic, 5 (50%) follicular variant, 1(10%) unspecified and 1 anaplastic. Each one classified as high risk. Locally advanced presentations are 50% and Metastatic Disease is 60%. Lung Metastasis 5(50%) and brain 2(20%) was the most frequent organ affected, elsewhere, liver 1 case (10%). One patient (10%) developed a second primary tumor: lung adenocarcinoma during the follow-up. Tracheostomy was the surgical procedure most used 7 cases (70%) follow of total Thyroidectomy 3(30%) with Radical Modified Neck Dissection (RMND) plus External Radiotherapy 1 (10%); with larynx and tracheal shave 1 (10%), with I-131 2 patients (20%); palliative care 1(10%) case and 1(10%) with Sorafenib. Progressive failure respiratory was the principal cause of death, 7 cases (70%) 2 cases (20%) asphyxia for progressive laryngotracheal obstruction and 1 cases for multi organ failure for multiple organ metastasis. The relation to overall survival 6 patients (60%) dead before the six months, 3 cases (30%) dead between 12-18 months and only 1(10%) patient lived more of 60 months.
Conclusion: This small series show that the deaths for the follicular variant of papillary thyroid cancer, in men of fifth decade of life, classified as high risk, were due to progressive failure respiratory secondary to lung metastasis and most occurred before of six months.
Death thyroid; Metastasis thyroid cancer
The incidence of thyroid cancer in the last decade has increased for early diagnosis [1]. However, death by this disease through long time has not change yet [2].
The biological behavior of thyroid cancer is the most indolent as death cause [3] but several researches have reported that different histology subtype as Insular, Tall cell tumor and anaplastic tumor are the most aggressive biologically [4]. Other side, classic papillary thyroid cancer, well differentiated, to long time, can change his behavior, and lost little too little the differentiation and finally change to undifferentiated, with all aggressive spectrum and change the prognosis of the patient of good prognosis to worse prognosis [5]. Nikiforov, et al. [6] reported the impact of the panel genetic mutation in thyroid cell that can predict the biology in a cytology test as preoperative molecular markers. Patients with multiples genetic mutation can develop generally older season over 50 years with larger tumor than 4 cm. The most common presentation is as Extra thyroid spread with invasion of local and regional structure associated with significant higher mortality. On the other side, death for locally advanced or metastatic disease are the principal cause, and represents an important challenge to select the best therapeutic approached [7].
The aim of this study is to present the cause of death in the patients with thyroid cancer.
Descriptive study, series of cases - We have reviewed the file records of patients with diagnosis of thyroid cancer, over study period. We have selected the patients that die to decide the death cause in each case and to know clinical characteristics of the patients, histological subtype of thyroid cancer as World Health Organizations (WHO) [4] risk factor classification using AGES stage system [8] and therapeutic approached. The univariable review was realized with frequency and percentage. The overall survival was done by using Kaplan-Meier.
The file records of 250 patients with thyroid cancer diagnosed were reviewed, 10 patients die which represent 4%. Male 7(70%), female 3(30%) (Figure 1). The papillary thyroid cancer was 9 cases (90%), 3(30%) classic, 5(50%) follicular variant, 1 case (10%) unspecified, only 1 case (10%) anaplastic (Figure 2). 10 patients (100%) classified as a high-risk factor using AGES classification, the size tumor greatest to 10 cm, central neck compartment clinically positive to illness (Photo 1A-C). Case (10%) with parapharyngeal space tumor (Photo 2A-B). Locally Advance presentation 5 patients (50%) (Photo 3). Metastasis disease 6 cases (60%), lung metastasis 5(50%) and brain 2(20%) was the most frequent organ affected, elsewhere, liver 1 case (10%)(Photo 4) (Figure 3). 1 case (10%) developed second primary tumor: lung adenocarcinoma during the follow up (Photo 5).

Figure 1: Patients death for thyroid cancer.
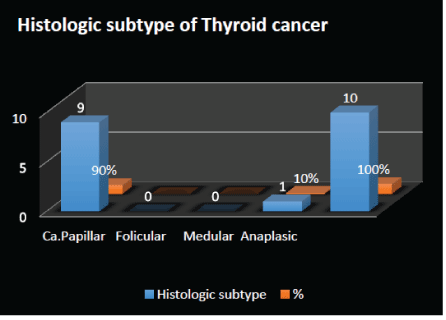
Figure 2: Histologic subtype of Thyroid cancer.
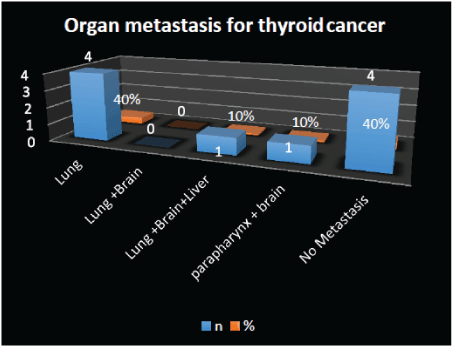
Figure 3: Organ metastasis for thyroid cancer.
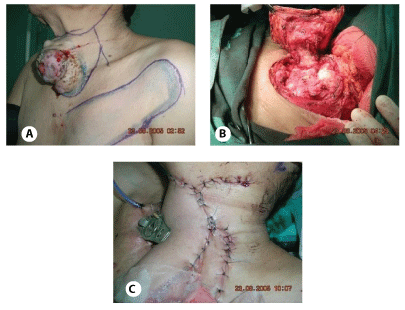
Photo 1: Female with papillary thyroid cancer locally advances.
1A: High Risk stratification
1B: Total thyroidectomy plus laryngotracheal shave
1C: Left modified radical neck dissection plus reconstruction plus radiotherapy.
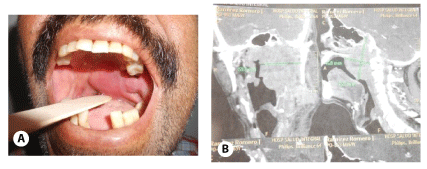
Photo 2: Case (10%) with parapharyngeal space tumor.
2A: Male, with papillary thyroid cancer in parapharyngeal space and brain metastasis.
2B: CT Scan Locally Advance presentation 5 patients (50%)
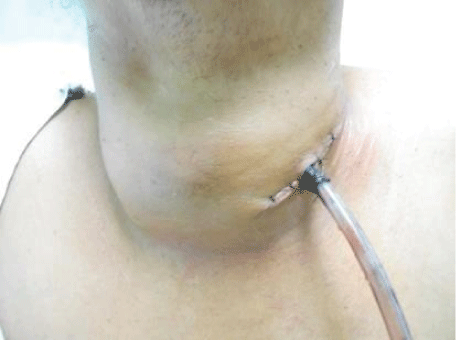
Photo 3: Female with papillary thyroid cancer locally advance. High Risk stratification. AGES
A: 56 years old
G: Female
E: Central compartment and soft tissue positive
S: Tumor size 10 cm diameter
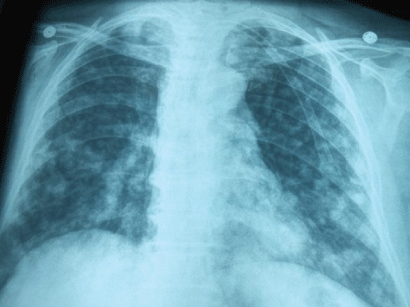
Photo 4: Female with papillary thyroid cancer with several lung metastasis.
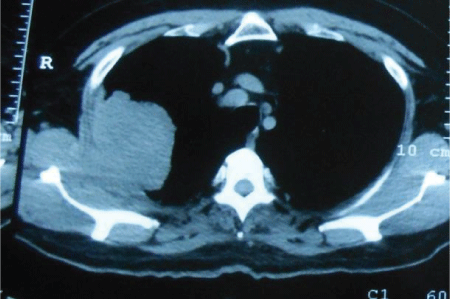
Photo 5: CT: Male with papillary thyroid cancer that developed a second primary tumor: lung adenocarcinoma.
One patient has papillary thyroid cancer with several local recurrences. Several surgical approached plus radioiodine ablation, without response. Developed multiple metastasis (Photo 6A-C). One patient with anaplastic tumor arrived to emergency room with fatal asphyxia for laryngotracheal obstructions due to gross tumor central and left compartment (Photo 7).
The several therapeutic approach used 7(70%) tracheostomy, total thyroidectomy 3(30%) with Radical Modified Neck Dissection (RMND) plus external radiotherapy 1; with larynx and tracheal shave 1, with I-131: 2 patients, palliative care 1 case and 1 with Sorafenib (Figure 4).
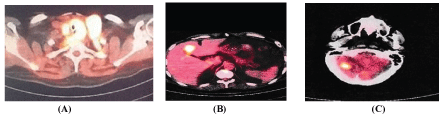
Photo 6A-C: One patient with papillary thyroid cancer with several local recurrences. Several surgical approached plus radioiodine ablation, without response. Developed multiple metastasis.
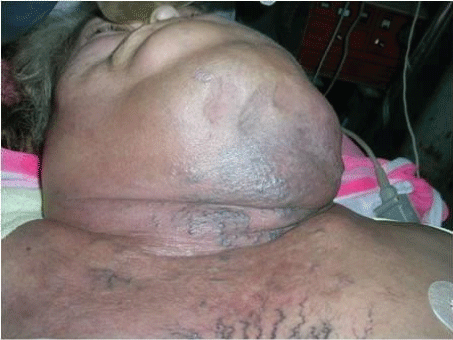
Photo 7: Female with anaplastic tumor, dead at emergency room.
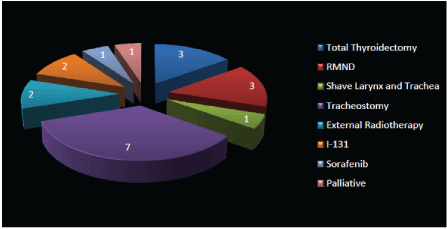
Figure 4: Several Therapeutic Approaches.
The relation to overall survival 6 patients (60%) dead before the six months, 3 cases (30%) dead between 12-18 months and only 1(10%) patient lived more of 60 months.
The cause of death was 7 cases (70%) progresive failure respiratory, 2 cases (20%) asphyxia for progresive laryngotracheal obstruction and 1(10%) cases for multiorgan failure for multiple organ metastasis.
The thyroid cancer is the most common in young women, generally diagnosed at early stage, [9] but the locally advanced or metastatic disease, frequently is a clinical presentation in older men just like ours results [10]. In this group, the tumoral biology is more aggressive and is associate with several risks factor of worse prognosis, relates with the tumor and the patients, so as ours research [11]. Tumor Size is >4 cm, extrathyroid extension, vascular, lymphatic and capsular invasion [12-14]. Risk stratification is the cornerstone for individualized thyroid cancer management. Based on American Thyroid Associated (ATA) this can estimates risk of recurrence or the risk specific disease mortality, however, other risk estimates can also significantly influence surveillance and therapeutic decision making [9,15]. Really, we know that papillary thyroid cancer has a high risk of lymph node metastasis, but, no clinical important impact at overall survival of the patients; accordingly, the TNM classification was not used. Several stages system to predict the risk to death for thyroid cancer has been used: AGES, AMES, MACES, for example, using similar or different variable, his predict risk to death one does not outperform the other. The majority of death by thyroid cancer occurred in the high risk group as ours results [16-19].
The papillary thyroid cancer is the most frequent histology subtype and the follicular variant which is typical of men has showed vessel invasion which explain the high risk of metastatic disease. Yang, et al. [20] describes that vessel invasion compares with follicular cancer has not statistical significance difference, however, Hagag, et al. [21] published the major prevalence of vascular and capsular invasion in follicular variant of papillary thyroid cancer, and this can explain the mayor number of locally advance and metastatic disease in our research [22].
While it is true, that the classic papillary thyroid cancer is the most biological behavior indolent, however less than 10% die of the disease, as ours results [23,24]. Current knowledge about of tumour heterogeneity develops through certain genetic or epigenetic change in tumor cell due to the influence of the tumour microenvironment. Diversity genetic within a single tumour is believed to result from multiples events that appearance of a driver mutation required for malignancy initiation [25,26]. After of the mutation in oncogenic genes, BRAF V600E and RET in thyrocytes cells are the most common detected in thyroid cancer with indolent behavior. Those have been used as preoperative molecular marker [6], but there are a group of patients with Well Differentiated Thyroid Cancer (WDTC) more aggressive, due to multiple genetic mutation NRAS, HRAS and KRAS [27,28].
Sometime, there are patients with a single gene mutation but associates with an epigenetic change that adds a aggressive behavior, due to the induction of genomic instability, anyway that a poor differentiate thyroid cancer or undifferentiated may develop from WDTC after acquiring further alteration [5].
Every one of this aspect have a clinical presentation that permit us understand: Why the early recurrence? Why multiple recurrence? Why regional invasive behavior? multiple metastasis disease and finally the no respond to the treatments in patients that die by thyroid cancer.
All those biomarker are very important tool in the assessment of the patients with thyroid cancer, however, are very expensive and to need an expertise team to develop [29]. We understand the great important to our patients but the high economic cost is not possible to undeveloped country.
The anaplastic is the most heterogenous of all the thyroid cancer subtype. Is typically widely invasive tumour that extent into the thyroid parenchyma and to the surrounding tissue or structure of the neck [26]. This demonstrates a heterogenous set of genetic alteration, TP53 gene mutation is characteristic for anaplastic thyroid cancer with a prevalence of 70% among cases. This mutation is a late molecular event in the thyroid carcinogenesis [30]. Morphologically it can simulate a fibrous sarcoma, undifferentiated pleomorphic sarcoma, angiosarcoma or rhabdomyosarcoma [4]. It is the less frequent as our result but of frequently diagnose in locally advance stage, generally with gross tumor invading laryngotracheal structure with asphyxia by obstruction as clinical presentation. The emergency tracheotomy should be done by surgeon with a lot experience [31,32].
The most frequent clinical presentation of thyroid cancer is a thyroid nodule, but, several research has reported different form of presentation like parapharyngeal space tumor with lung, brain and liver metastatic disease, this is the classic presentation of our patient with advance thyroid cancer that progress to death [33-36]. The lung metastatic disease causes respiratory failure leading to death, the same as expressed in our series [37].
One of our patients developed a lung adenocarcinoma, in spite of two years follow of thyroid cancer advanced without active disease after total thyroidectomy plus central neck dissection plus iodine ablation. Between 5-10% with head and neck cancer can develop a second primary tumor that is the cause of death in a short time [38].
The best choice of treatment modality always represents a challenge in each patient with locally advance or metastatic disease. Greatest surgical approaches has been describe when thyroid cancer invading laryngotracheal structure but with a high impact in the quality of life of patient [39]. But in many cases tracheostomy is the best procedure [40]. There is a group of patient that prefers palliative care or external beam radiation with hemostatic intention if ulcerative lesions are present [41]. Actually the therapy with antibody monoclonal is an alternative for metastatic diseases [42] the use of Sorafenib has showed to stop the progression of diseases with a better quality of life [43].
This small series shows that the deaths for the follicular variant of papillary thyroid cancer, in men of fifth decade of life, classified as high risk, were due to progressive failure respiratory secondary to lung metastasis and most occurred before of six months.
Download Provisional PDF Here
Article Type: CASE SERIES
Citation: Medrano F, Matus GR, Quezada C, Carmona G (2018) Death by Thyroid Cancer: Series of Cases. January 2003-December 2017. Int J Endocrinol Metab Disord 4(2): dx.doi.org/10.16966/2380-548X.149
Copyright: © 2018 Medrano F, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Publication history:
All Sci Forschen Journals are Open Access