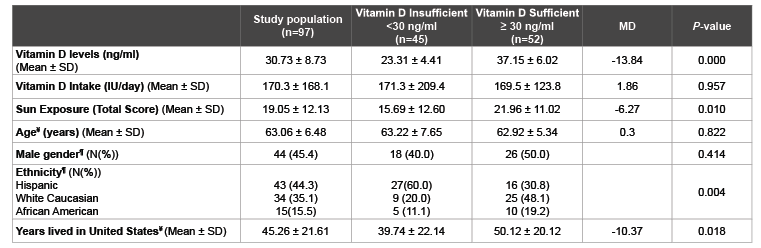
Table 1: Comparison of Vitamin D Related Variables and Sociodemographic Characteristics between Vitamin D Insufficient and Sufficient Participants
¥
Independent T-tests; ¶
Chi-squares; SD: Standard Deviations; N: Number; MD: Mean Difference

Johanna Lopez1,2 Adriana Campa1 Fatma G Huffman1 Juan P Liuzzi1 Tan Li3 Ana H Martinez4 Serena M Ferris4 Dott Carungcong4 Ashar Farooqi4 Ammar Rasu4 Ana M Lopez Medrano4 Steven E Atlas4 Eduard Tiozzo4 Janet Konefal5 Judi Woolger5 John E Lewis4*
1Department of Dietetics and Nutrition, Florida International University, Miami, FL 33174, USA*Corresponding author: John E. Lewis, Department of Psychiatry & Behavioral Sciences, University of Miami Miller School of Medicine, 1120 NW 14th Street Suite #1474, Miami, FL 33136 USA, Tel: +1 305 243 6227; Fax: +1 305 243 1619; E-mail: jelewis@miami.edu
Objective: The purpose of this cross-sectional analysis is to determine the proportion of vitamin D insufficient individuals and correlates of vitamin D status in an older adult population (≥ 55 years of age) living in Miami-Dade, Florida.
Methods: Participants (n=97) completed assessments that included vitamin D serum levels, surveys to evaluate vitamin D intake, sun exposure, and other variables. Spearman’s correlations, independent t-tests, chi-squares, and multiple linear and logistic regression were used to examine the relationship between vitamin D status and explanatory variables.
Results: The proportion of individuals with vitamin D deficiency (25-hydroxyvitamin D <0 ng/ml), insufficiency (20-30 ng/ml) and sufficiency (≥ 30 ng/ml) were 10.3%, 36.1% and 53.6%, respectively. Vitamin D status correlated significantly with higher vitamin/mineral supplement use (P=0.014), and lower percent fat mass (P=0.008) in linear regression models, and increased sun exposure (P=0.016) in logistic regression models. However, ethnicity was consistently an independent predictor of vitamin D levels in both analyses (P=0.023 and P=0.010, respectively), with Hispanics being at higher risk of insufficiency.
Conclusions: Since vitamin D deficiency is linked to health risk factors, it is important that health professionals become aware of the connections of vitamin D status with intake, bioavailability, and skin synthesis, to identify those at risk and develop and plan pertinent interventions to prevent and correct deficiency.
Vitamin D insufficiency; Vitamin D intake; Sun exposure; Elderly; Ethnicity
25(OH)D: 25-hydroxyvitamin D; BDI: Beck Depression Inventory; BRFSS: Behavioral Risk Factor Surveillance System; BIA: Bioelectrical Impedance; BMI: Body Mass Index; CI: Confidence Interval; d.f: Degrees of Freedom; DSM-IV: Diagnostic and Statistical Manual of Mental Disorders Fourth Version; DRIs: Dietary Reference Intakes; FFM: Fat Free Mass; FM: Fat Mass; FFQ: Food Frequency Questionnaire; HHHQ: Health Habits and History Questionnaire; HC: Hip Circumference; ICMA: ImmunoChemilumino Metric Assays; IOM: Institute Of Medicine; IRBs: Internal Review Boards; IPAQ: International Physical Activity Questionnaire; IU: International Unit; MD: Mean Difference; MET: Metabolic Equivalents; NHANES: National Health and Nutrition Examination Survey; OR: Odds Ratio; SPMSQ: Short Portable Mental Status Questionnaire; SD: Standard Deviation; SE: Standard Error; UVB: Ultraviolet B; WC: Waist Circumference; WHR: Waist-to-Hip Ratio; β: Beta Coefficient
Vitamin D deficiency in the elderly has been linked to factors associated with disability, including cognitive impairment, poor physical performance, and increased risk of falls and fractures [1-7]. The elderly is at higher risk of vitamin D deficiency as shown by National Health and Nutrition Examination Survey (NHANES) 1988-1994 data that indicated serum vitamin D levels were lower among adults 65 years and older, compared to middle aged (40-59 years of age) and younger adults (18-29 years of age) [8,9]. Vitamin D is considered a steroid hormone obtained naturally from dietary sources and skin synthesis and stored in muscle and fat tissue. The body can use muscle and fat reserves independent of sun exposure and dietary intake, only when vitamin D concentrations are lower than 20 ng/ml [10-12]. For older adults, reaching this vitamin D threshold is difficult; they become more dependent on their vitamin D reserve, as they encounter more barriers for adequate dietary and skin sources, which place them at higher risk of deficiency and insufficiency. This may be a consequence of a complex interplay between age-related factors that affect dietary intake, bioavailability, and skin biosynthesis [10-12].
In the United States, very few foods contain significant amounts of vitamin D. For those who are not taking adequate amounts of vitamin D through diet or supplement use, sunlight would often be their main source of vitamin D [13,14]. Vitamin D is mostly synthesized in the skin when ultraviolet B (UVB, 290–315 nm) light from the sun causes a photochemical rearrangement of 7-dehydrocholesterol to pre-vitamin D3, which then undergoes thermal isomerization into vitamin D3 [13,15,16]. The skin’s ability to synthesize vitamin D depends on the amount of solar radiation reaching the biosphere that depends on latitude, season, and time of day [15,16]. Since 90-95% of vitamin D comes from skin synthesis, it would be expected that the prevalence of vitamin D deficiency and insufficiency to be lower in a populations where sunlight for synthesis is available year round [17,18]. Thus, it has been assumed that vitamin D deficiency and insufficiency is less prevalent in populations living at lower latitudes and/or where seasonal variations are not as pronounced like in South Florida, one of the southernmost regions of the United States (latitude 25.46N) where the weather is sunny and warm all year [19]. However, research has shown a considerable amount of vitamin D deficient and insufficient individuals. A previous study reported a prevalence of 38-40% of hypovitaminosis D (25-hydroxyvitamin D or 25(OH)D<20 ng/ml) in adults (≥ 18 years old) living in South Florida [19]. Lagari et al. [20] found that 35% of participants in a study conducted in Miami-Dade were vitamin D insufficient (25(OH)D<30 ng/ml). Another study conducted in Boca Raton, Florida, evaluated vitamin D status in a community dwelling elderly population (≥ 60 years old) and found an occurrence of vitamin D insufficiency (25(OH)D<25 ng/ml)of 15.6% [21].
The determinants of vitamin D status in the elderly population in Miami–Dade have not been well studied. This is important because Miami occupies the fourth place among cities that are graying the fastest, and it is the top county for elders, with 14.9% of the Miami-Dade population being over the age of 65 [22]. Understanding the factors that affect vitamin D status in this growing population of older adults, who have an increased opportunity for skin biosynthesis via sun exposure, is critical to promote public health initiatives aimed to correct and prevent nutritional deficiencies. Therefore, the purpose of this cross-sectional analysis was to evaluate correlates of vitamin D insufficiency in older adults living in Miami-Dade.
This study was approved by the IRBs of Florida International University (13-0390) and the University of Miami (20120195).
The study enrolled 101 participants, and four were excluded due to missing data (n=97). Participants for this study were recruited from the parent study, a double-blinded randomized placebo-controlled clinical trial that examined the effects of vitamin D supplementation on vitamin D level, bone formation, resorption, and mineral density, flexibility, and balance in the elderly, which was conducted at the University of Miami. Briefly, each potential participant was screened over the phone by administering the Short Portable Mental Status Questionnaire (SPMSQ) [23] to evaluate mental functioning (allowed up to 2 errors) and questions regarding general inclusion criteria such as age, and medical, pharmacological and general exclusions. Inclusion criteria for this study included: (1) Men and women age 55 or older; (2) English and Spanish speakers; (3) Community-dwelling; (4) Ability to give informed consent; (5) Ability to perform motor tasks without aid; and (6) Participating in the parent study. All participants in this study were required to sign IRB approved informed consents for both the University of Miami and Florida International University.
Vitamin D Status: Vitamin D status was measured by serum 25-hydroxyvitamin D (25(OH)D). Fasting venous blood (15 ml) was collected from every participant by a certified phlebotomist in the morning after fasting. The samples were sent the same day for analysis to LabCorp (2700 N. 29th Ave, Suite 203A, Hollywood, Fl 33020) that used immunochemiluminometric assays (ICMA) on the DiaSorin Liaison instrument to assess 25(OH)D. This is a highly automated test that measures total 25(OH)D, and it has been widely used by others [24].
Sociodemographics, health history, health risk behaviors and medications: Sociodemographic and health-related data were collected using questionnaires developed by staff of the parent study that asked about gender, marital status, race/ethnicity, socioeconomic status, education, income, current medical diagnoses, medication/ supplement use and health risk behaviors such as coffee, alcohol, and tobacco use.
Vitamin D Intake: Vitamin D intake was assessed using a vitamin D and calcium specific short food frequency questionnaire (FFQ) developed by Blalock et al. [25], which is based on the Block-National Cancer Institute Health Habits and History Questionnaire (HHHQ). It includes only 23 foods and beverages that were identified in the HHHQ as rich in either vitamin D, calcium or both. The short FFQ is associated with a database; therefore, allowing the estimation of nutrient intakes for the reported portion size. The raw values were multiplied by the frequencies to calculate the amount of vitamin D and calcium per day using Microsoft Access. Blalock et al. [25] validated this tool and the vitamin D and calcium intakes estimated from this short FFQ were significantly correlated with estimates from the 7-day food diary (r=0.72 and 0.66; respectively) and the original HHHQ (r=0.65 and 0.66, respectively). Positive predictive values of 100% for vitamin D and 91.7% for calcium were reported for the short instrument in its ability to identify those with low intakes as based on the 7-day food record.
Sun exposure: Sun exposure was assessed using a questionnaire developed by Hanwell et al. [26]. This tool measures the amount of time spent outdoors and the amount of skin exposed for every day of the week using a scoring system. The original questionnaire by Hanwell et al. [26] was developed for hospital workers in Southern Italy and designed to report the sun exposure within the last week by recording time outdoors (<5 min; 5-30 min; >30 min) and amount of skin exposed (hands and face; hands, face and arms; hands, face and legs; bathing suit). The amount of time spent outdoors was multiplied by the amount of skin exposed for every day of the week. The sum of the seven days was used as the sun exposure scores. These researchers found that the sun exposure score was significantly correlatedwith serum vitamin D levels during the summer, which was mostly explained by the time in the sun than by the amount of skin exposed. Other researchers have validated this tool in other populations in South Florida [27,28].
Physical activity levels: Physical activity level was measured using the International Physical Activity Questionnaire (IPAQ) [29], which provides individual domain-specific scores for walking, moderate-intensity and vigorous-intensity activity within the domains of work, transportation, domestic chores and gardening, and leisure-time. To measure the volume of activity, each type of activity was weighted by its energy requirement defined as the MET-min. METs are metabolic equivalents that are computed for each specific activity using defined formulas, and then multiplied by the minutes performed to derive MET-minute scores, which are equal to kilocalories for a 60 kg person [29].
The IPAQ was developed to derive comparable measurements of physical activity in international settings; therefore, being suitable for use in different languages and cultural contexts like in an ethnically-diverse city as Miami [30,31]. It has also been validated in different age groups against objective measures of physical activity, physical fitness, and health outcomes [32]. For instance, it was validated in an adult population (mean ± SD age: 40.7 ± 10.3 years) and found to have a strong positive relationship with an activity monitor and a physical activity log (rho=0.55, P<0.001) [33]. It was also validated and used in a population of elderly men and found to be reliable (test-retest reliability of 0.95) and valid when compared against pedometer use and the physical activity log, and had similar results in cohorts of postmenopausal women [34,35]. Other studies have validated the IPAQ and used it successfully in populations of middle-aged and older adults and adults with different diseases, such as schizophrenia and breast cancer [31,36-39]. The IPAQ correlated with measurements to other physical activity assessment methods such as the 2001 Behavioral Risk Factor Surveillance System (BRFSS) physical activity, accelerometers, and energy expenditure as measured by doublylabeled water, among others [32,40-43].
Depressive symptoms: Depressive symptoms were assessed using the Beck Depression Inventory (BDI-II), which is a widely validated and used tool that evaluates the existence and severity of depressive symptoms based on the DSM-IV [44]. It is a self-report, 21-item instrument in which each item corresponds to a symptom of depression and has a four-point scale ranging from 0 to 3, except for two items that have seven options to indicate decrease or increase in appetite and sleep. The score of all items are summed to give a single score. A total score of 0-13 is considered minimal, 14-19 mild, 20-28 moderate, and 29-63 severe depressive symptoms [44]. Construct validity has been assessedon its ability to differentiate nondepressed and depressed patients (0.92 for outpatients and 0.93 for college students). Test-retest reliability was found to be significant at 0.93. This tool has been used in population ages 13-80 years [44-47]. In an elderly population, BDI-II has good internal consistency (0.86), and has been positively correlated with other measures of depression, measures of stress, anxiety, and negatively with well-being. Research with this instrument has shown no statistically significant effects for ethnicity, gender, and age on BDI-II scores [48]. In case a participant reported suicidal ideation, the investigators in this study contacted the study physician and/or University of Miami Mental Health Department, immediately.
Weight was measured using an electronic balance. Participants were asked to remove shoes and heavy outer garments like coats and to stand in the center of the balance so that the weight was distributed evenly on both feet. Height was measured using a stadiometer with a movable headpiece. Participants were asked to stand with their back to the height rule with their feet together, while the back of the head, back, buttocks, calves, and heels touched the upright tape. The participant was asked to look straight ahead so that the ear canal was level with the cheek bone. The headpiece was lowered so that the hair was pressed flat. Weight and height were recorded to the nearest 0.1 kg and 0.1 cm, respectively. Body mass index (BMI) was calculated using the formula: body weight (kg)/ height (m2 ) and accordingly, participants were classified into categories based on the National Heart, Lung and Blood Institute recommendations: underweight (<18.5 kg/m2 ), normal weight (18.5-24.9 kg/m2 ); overweight (25-29.9 kg/ m2 ), and obese (>30 kg/m2 ).
Waist circumference (WC) was measured at the umbilicus and hip circumference (HC) was measured at the broadest circumference below the waist. Both of these measurements were done using a flexible but not stretchable measuring tape in three replicates to ensure reproducibility. The average of the three values was used to calculate waist-to-hip ratio (WHR) by dividing WC by HC. Body composition was measured using bioelectrical impedance (BIA), to determine % lean (fat-free, %FFM) and fat mass (%FM).
Statistical analyzes were performed on 97 participants. Data were analyzed using frequencies, percentages, ranges, means, and standard deviations. Variables were checked for non-normality and if necessary, they were transformed to achieve a normal distribution. Spearman correlations between two or more categorical variables were performed to evaluate the relationship between vitamin status, vitamin D intake, sun exposure, sociodemographic characteristics, health-related variables, physical activity, depressive symptoms, and anthropometries. Independent t-tests or chi-squares were used to compare vitamin D insufficient and sufficient categories in vitamin D intake, sun exposure, sociodemographic characteristics, health-related variables, physical activity, depressive symptoms, and anthropometries. Multiple linear regressions were used to examine the effect size and the change in vitamin D levels expected from a one-unit change in a set of independent explanatory variables. Multiple logistic regression were used to examine the relationship between vitamin D insufficiency and a set of explanatory variables to identify independent correlates of vitamin D status and calculate adjusted odds ratio. Collinearity was assessed, and variables that inter-correlated with other independent variables were removedfrom the regressions. The significance level was set at α=0.05, and statistical analyses were performed using SPSS 21.
A total of 97 participants aged 55 years or older were included in this analysis. The mean ± SD of 25(OH)D concentration was 30.73 ± 8.73 ng/ml and ranged from 10.00 to 59.80 ng/ml. Ten percent, 36%, and 54% of the participants were vitamin D deficient (25(OH)D<20 ng/ml), insufficient (20-30 ng/ml) and sufficient (≥ 30 ng/ml), respectively. Since the number of vitamin D deficient individuals was low, vitamin D deficient and insufficient individuals were grouped into vitamin D insufficient (<30 ng/ml) for data analysis. The mean ± SD age was 63.06 ± 6.48 and ranged from 55-87 years (Table 1). Participants were 63.9% and 36.10% over the age of 60 and 65 respectively, 45% were males, and 44% were Hispanics, 35% white Caucasians, and 15.5% African-Americans. Comparison of vitamin D status groups revealed no significant difference in age, gender, and the proportion of participants who were married, unemployed and with less than college education and income of <$30,000 per year (data not shown).
A significant difference in sun exposure (mean difference: -6.27, P=0.01) was observed between vitamin D categories, with those who were insufficient having lower sun exposure scores (Table 1). No significant difference was found in vitamin D intake between the categories (P=0.957). Furthermore, only 2.1% of the participants met the Dietary Reference Intake (DRI) of 600 IU/day (data not shown).

Table 1: Comparison of Vitamin D Related Variables and Sociodemographic Characteristics between Vitamin D Insufficient and Sufficient Participants
¥
Independent T-tests; ¶
Chi-squares; SD: Standard Deviations; N: Number; MD: Mean Difference
A comparison between vitamin D status groups revealed several significant differences in potential determinants of vitamin D levels. Those who were vitamin D insufficient lived in the United States significantly fewer years (mean difference:-10.37, P=0.018). Ethnicities were also significantly different between vitamin D groups. More Hispanics and less white Caucasians and African Americans were in the vitamin D insufficient group (P=0.004) (Table 1). A one-way ANOVA comparison between ethnic groups on vitamin D levels showed significant differences between ethnicities (F (d.f.): 6.27 (3), P=0.001). Hispanics had lower vitamin D levels (mean ± SD: 27.61 ± 7.75) than White Caucasians (mean ± SD: 33.37 ± 7.73, P=0.015) and African-Americans (mean ± SD: 35.77 ± 9.60, P=0.007) (Table 2).
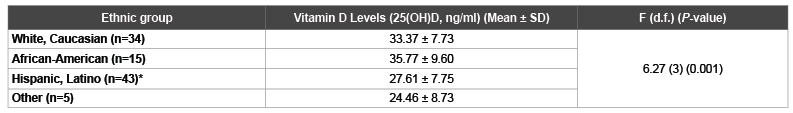
Table 2: One-Way ANOVA Comparison of Vitamin D Levels between Ethnic Groups
*
Post Hoc Comparisons of Hispanic, Latino vs. White, Caucasian P=0.015 and Hispanic, Latino vs. African-American P=0.007; SD: Standard Deviation;
d.f.: degrees of freedom
Sixty-four percent of participants who were vitamin D insufficient used prescription medications compared to 85% of those who were sufficient (P=0.033) (Table 3). Also, those in the vitamin D insufficient group were taking significantly fewer prescription medications than those in the vitamin D sufficient group (mean difference: -0.94, P=0.033). Thirty-eight percent of those who were vitamin D insufficient reported taking vitamin/ mineral supplements compared to 54% of the sufficient group, but this difference was not significant (P=0.153).
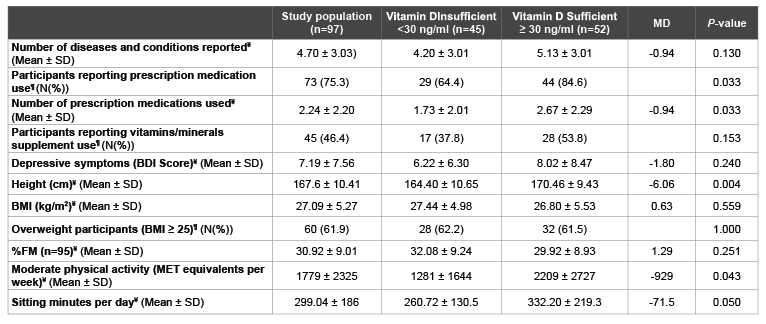
Table 3: Comparison of Vitamin D Levels, Vitamin D Intake, Sun Exposure and Other Potential Correlates between Vitamin D Status Groups
¥
Independent T-tests; ¶
Chi-squares; SD: Standard Deviations; N: Number; MD: Mean Difference; BDI: Beck Depression Inventory; BMI: Body Mass Index;
FM: Fat Mass; MET: Metabolic Equivalents
Physical activity was significantly different between groups with those who were vitamin D insufficient having significantly less moderate physical activity (mean difference: -928.61, P=0.043) (Table 3). Height was the only anthropometric variable that was significantly different between groups, with those who were vitamin D insufficient being shorter (mean difference: -6.06, P=0.004). BMI and %FM were higher in the vitamin D insufficient group, but the difference was not significant (mean difference: 0.63, P=0.559, and mean difference: 1.29, P=0.251, respectively).
Spearman correlations showed a significant association between vitamin D categories and potential correlates of vitamin D status (Table 4). Significantly different variables between groups were also significantly correlated with vitamin D status. Sun exposure (r=0.274, P=0.007), years lived in the Unites States (r=0.272, P=0.007), number of participants taking prescription medications (r=0.233, P=0.022), and number of prescription medications (r=0.234, P=0.021) were positively correlated with vitamin D status, while ethnicity (r=-0.365, P=0.000) and height (r=- 0.298, P=0.003) were negatively correlated. Although vitamin D status did not significantly correlate with vitamin D intake (r=0.05, P=0.728), it was positively correlated with vitamin/mineral supplement use (r=0.277, P=0.006). Lastly, vitamin D status and a negative association with BMI (r=-0.220, P=0.030), and %FM (r=-0.263, P=0.010).
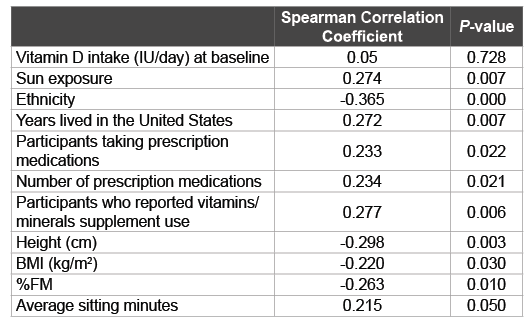
Table 4: Vitamin D Status Correlation with Vitamin D Intake, Sun Exposure,
and Other Potential Predictors
Spearman Correlations between vitamin D status groups (Vitamin D
insufficient=0; Vitamin D sufficient=1) and continuous/categorical variables.
BMI: Body Mass Index, FM: Fat Mass.
To explain the effect of sun exposure and dietary intake on vitamin D levels, multiple linear regressions were used (Table 5). Vitamin D intake had no significant effect (B (SE): 0.00 (0.01), P=0.648), while sun exposure significantly predicted 4% of the variation in vitamin D levels (B (SE): 0.17 (0.07), P=0.017). In this model, vitamin D levels were expected to increase by 0.17 ng/ml for every unit increase in sun exposure. Adding ethnicity to the model showed that sun exposure was no longer a predictor of vitamin D levels (P=0.052). In this model, non-Hispanics compared to Hispanics would be expected to have 5.02 ng/ml higher vitamin D levels (B (SE): 5.02 (1.70), P=0.004). Thus, sun exposure and ethnicity explained 12% of the variation in vitamin D levels.
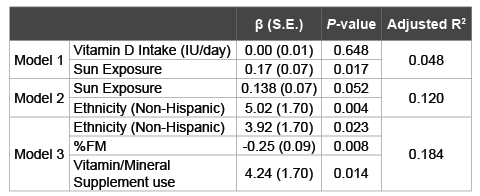
Table 5: The Relationship between Vitamin D Status and Potential
Correlates in Multiple Linear Regressions
Stepwise Multiple Linear Regressions: Dependent variable: Vitamin D
levels (ng/ml). Model 1: Vitamin D Intake, Sun exposure; Model 2: Model 1
+ Ethnicity; Model 3: Model 2 + Years lived in the US, number of participants
taking prescription medications, number of prescription medications,
height, moderate physical activity, average sitting time per day, education,
vitamin/mineral supplement use, BMI, %FM, age, gender, marital status,
employment, income, number of disease, coffee use, alcohol use, smoking,
weight, calcium intake, depressive symptoms, β: Beta Coefficient; S.E.:
Standard Error; CI: Confidence Interval; FM: Fat Mass.
Ethnicity remained a significant predictor of vitamin D levels after adding variables that (1) were significantly different between vitamin D status categories, (2) correlated significantly with vitamin D status; and/ or (3) have been shown in previous research to be significantly correlated with vitamin D levels (B (SE): 3.92 (1.70), P=0.023). In this fully adjusted model, ethnicity, %FM (B (SE): -0.25 (0.09), P=0.008), and vitamin/ mineral supplement use (B (SE): 4.24 (1.70), P=0.014) explained 18.4% of the variation in vitamin D levels. For every unit increase in %FM, vitamin D levels were expected to decrease by 0.25 ng/ml. Furthermore, compared to those who did not use supplements, those who reported taking vitamin/ mineral supplements were expected to have 4.24 ng/ml higher vitamin D levels.
Multiple logistic regressions showed that higher sun exposure scores predicted vitamin D levels with those with higher scores being 1.05 times significantly more likely to be vitamin D sufficient (OR: 1.05, 95% CI: 1.01; 1.09, P=0.013) (Table 6). In the fully adjusted model, sun exposure relationship to vitamin D levels remained about the same (OR: 1.05, 95% CI: 1.01; 1.10, P=0.016). In addition, this model revealed that Hispanics were 0.30 times less likely to be vitamin D sufficient (OR: 0.30, 95% CI: 0.12; 0.75, P=0.010).
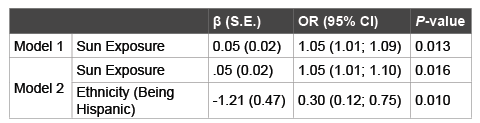
Table 6: The Relationship between Vitamin D Status and Potential
Correlates in Multiple Logistic Regression
Forward Logistic Regression: Dependent Variable: Vitamin status (0=<30
ng/ml; 1=≥ 30 ng/ml). Model 1: Vitamin D intake, Sun Exposure. Model
2: Model 1 + Ethnicity, Years lived in the US, number of participants
taking prescription medications, number of prescription medications,
height, moderate physical activity, average sitting time per day, education,
vitamin/mineral supplement use, BMI, %FM, age, gender, marital status,
employment, income, number of disease, coffee use, alcohol use, smoking,
weight, calcium intake, depressive symptoms; β: Beta Coefficient; SE:
Standard Error; OR: Odds Ratio; CI: Confidence Interval.
The proportion of vitamin D deficient (25(OH)D<20 ng/ml) and insufficient (25(OH)D<30 ng/ml) individuals in this group of healthy community-dwelling older adults (>55 years old) living in Miami-Dade was 10% and 36%, respectively. This was lower than that reported for the general United States adult and older adult population [11,49]. NHANES 2005-2006 revealed that 41.6% of United States adults (18 years or older) had deficient vitamin D levels, defined as 25(OH)D<20 ng/ml [11,49]. A previous study conducted in South Florida showed a prevalence of 38- 40% of hypovitaminosis D (25(OH)D< 20 ng/ml) in adults (≥ 18 years old) [19]. In older adults, NHANES 1988-1994 data indicated that 23% of adults 65 years and older were vitamin D deficient (25(OH)D<20 ng/ ml), and 62.5% were vitamin D insufficient (25(OH)D<30 ng/ml) [8,9]. Several reports from The Health ABC cohort study described 65-68% of adults over the age of 70 as vitamin D insufficient [50-52].
Vitamin D deficiency and insufficiency in our study group was less than half of that previously reported for the general United States older adult population. However, our results are similar to that reported by Lagari et al. [20] who observed vitamin D insufficiency in 35% of the older adult population (>65 years of age) participating in a study in MiamiDade. Furthermore, in older adults (≥ 60 years of age) living in Boca Raton, Florida, Smolar et al. [21] found a prevalence of 15.6% vitamin D insufficiency, defined as 25(OH)D<25 ng/ml. Using the same cut-of value, we found a higher occurrence with 27% of our study participants being vitamin D insufficient.
Despite the lower occurrence of vitamin D insufficiency in this study compared to other studies and the general United States older adult population, it is still a public health concern and identifying risk factors is critical to developing effective interventions. As expected, vitamin D intake was not significantly correlated with vitamin D status in our study, and our findings are supported by other studies that have previously shown that dietary intake has minimal contribution to vitamin D status [53,54]. The Institute of Medicine (IOM) committee reported in 2010 that in order to maintain serum 25(OH)D above 20 ng/ml, which was deemed necessary to preserve bone health, the intake of vitamin D should be 600 International Units (IU) per day for adults below the age of 70 and 800 IU per day for adults over the age of 70, with a tolerable upper limit of 4000 IU per day [55-58]. Although these are the established Dietary Reference Intakes (DRIs) for vitamin D, the Endocrine Society recommends adults over the age of 18 should intake between 1,500-2,000 IU per day, with an upper limit of 10,000 IU per day, to maintain 25(OH)D between 40-60 ng/ ml to conserve physiological functions that go beyond bone health [59]. However, in our study only 2% of the participants met the DRI of 600 IU per day (not accounting from vitamin D from supplement use) and the mean ± SD was 170.33 ± 168.06, with a range of 4.7–1,412.3 IU per day. Inadequate dietary intake of vitamin D is likely due to the limited availability of vitamin D rich foods in the daily diet of the elderly. Good sources of vitamin D include fatty fish such as salmon, tuna, and sardines, fish oil, egg yolks, organ meats, mushrooms, and fortified foods like milk and dairy products, breakfast cereals, bread, margarine, spreads, and vegetable oils [16,60]. This may be aggravated in the elderly by age-related decline in food intake that results from an interplay between physiological and psychological factors, such as changes in taste/smell, chewing/ swallowing problems, polypharmacy, hormonal factors regulating satiety, comorbidities, and age-related lactose intolerance that limits the intake of fortified milk products [49,61-66].
Supplement use may compensate for the lack of vitamin D intake from foods. Use of vitamin D supplements has increased over time in adults, with 26% of adults reporting using vitamin D supplement in NHANES 1988-1994, 25% in NHANES 1999-2002, and 37% in NHANES 2003- 2006 [67]. However, low vitamin D intakes from food and supplements have been reported in older adults and associated with low serum vitamin D [17,18,68]. Although using vitamin D and calcium supplements 2 weeks before the study were exclusion criteria in our study, we found that 46% of participants who reported a history of vitamins/minerals use, 37.8% were vitamin D insufficient and 53.8% were sufficient, but this difference was not significant. However, we did find that using vitamin/ mineral supplements was a significant and independent predictor of vitamin D levels in adjusted multiple linear regressions, with those using supplements having higher 25(OH)D. In addition, we found significantly more vitamin D sufficient participants who reported taking prescription medications and a higher number of prescription medications compared to the insufficient group, and these variables had a significant association with vitamin D status. This relationship could be due to physicians recommending more vitamin D supplements to their elderly patients because of the increased public health awareness of the consequences of vitamin D deficiency in older adults. It could also be possible that those who are taking prescriptions are more likely to have access to healthcare or be more health conscious. Nonetheless, unlike vitamin/mineral supplement use, receiving prescription medication and a higher number of prescription medications were not predictors of vitamin D levels in neither multiple linear nor logistic regressions.
Other risk factors for vitamin D insufficiency are those that affect bioavailability. The presence of other comorbidities and behavioral factors, like liver, kidney, and gastrointestinal diseases, and the use of certain medications, tobacco, and alcohol, may also affect vitamin D metabolism and bioavailability [66]. However, our study excluded participants who had any diseases/conditions or were taking any medications that affected vitamin D metabolism and absorption and hence, its bioavailability. Thus, we could not assess their effect on vitamin D status in our sample. We found that the number of diseases and condition, health risk behaviors and history of diseases/conditions, which have been significantly associated with vitamin D deficiency in previous studies, were not significantly different between, associated with, or predictive of vitamin D status. Obesity was the only condition that we were able to assess its effect on vitamin D status in our sample [49-66]. In the older adult, a decline in metabolic rate and hormonal changes that occur during aging often result in weight gain, as seen in the growing prevalence of obesity among elderly in the United States [69,70]. Obesity has been correlated with low vitamin D status in the elderly probably due to sequestration of vitamin D by increased body fat [18,49,66]. In our sample, the mean ± SD of BMI was 27.09 ± 5.27 with 62% of participants being overweight (BMI ≥ 25). Even though vitamin D insufficient participants had higher BMIs, the number of participants who had a BMI ≥ 25 kg/m2 was lower, and neither was significantly different compared with those who were vitamin D sufficient. However, BMI was significantly correlated with vitamin D levels, with those with lower vitamin D levels having higher BMI, but this association was no longer significant when adjusting for covariates in regression models. Abdominal fat accumulation, as measured by WC and WHR, were also not significantly correlated with vitamin D levels, nor different between vitamin D groups. However, the %FM was higher in the vitamin D insufficient group, but this difference was not significant. Nevertheless, it had a significant negative correlation with vitamin D levels, with those with lower 25(OH)D having higher %FM. Also, %FM was found to predict vitamin D levels in linear regression models. Thus, body fat is a risk factor for vitamin D deficiency in this Miami-Dade older adult population probably by affecting bioavailability.
The other factor that may affect vitamin D status is skin biosynthesis. For those who are not taking adequate amounts of vitamin D through diet, sunlight would often be their main source of vitamin D [13,14]. It is believed that sunlight exposure of arms and legs for 5-15 minutes at midday during the summer months could produce about 3,000 IU of vitamin D [12]. However, the effectiveness of vitamin D synthesis in the skin is determined by skin thickness, 7-dehydrocholesterol content, skin pigmentation, amount of skin exposed, sunscreen use, length of sun exposure, and amount of solar radiation (which depends on the latitude, season, time of day, and time in the sun).
Decreased skin thickness and reduction of 7-dehydrocholesterol are two age-related factors that place elderly at high risk for vitamin D deficiency [71]. This is aggravated by decreased sun exposure, since most people avoid sunshine to stay cool, prevent skin aging, and reduce the risk of skin cancer by limiting the amount of time spent outdoors. Covering the skin with clothes and using sunscreen are common practices among the elderly, the latter interfering with UVB transmission to the skin [66]. Thus, sufficient exposure to sunlight is unusual with modern lifestyles, since most people live and work indoors [66]. A study in Europe showed that wearing short sleeves instead of long, and thus bearing arms as well as the face, are associated with better vitamin D status in older people living in sunnier climates [72]. Furthermore, outdoor activity is associated with vitamin D status as shown by an analysis of NHANES 1988-1994 data that revealed that those who were 60 years or older and participated in daily outdoor activity had optimal vitamin D levels (≥ 30 ng/ml) similar to young adults [73]. In our study, sun exposure was measured by assessing the amount of skin exposed and time spent outdoors during sunny hours, and we found that sun exposure was significantly higher in participants who were vitamin D sufficient. Our data also showed a significant positive correlation between vitamin D status and sun exposure scores. In addition, we found that sun exposure had an effect on vitamin D levels, explaining only 4% of vitamin D status variability, and this was no longer significant when adjusting for covariates. However, participants with higher sun exposure scores had higher odds of being vitamin D sufficient.
Furthermore, physical activity, which is often used as a surrogate for outdoor activity, has been positively associated with serum vitamin D levels such that lower levels of serum vitamin D have been correlated with physical inactivity in middle-aged and older adults [18,74]. We only found that moderate physical activity was significantly different between vitamin D categories, with those insufficient having less activity than those who were sufficient. However, none of the physical activity variables were significantly correlated with or predictive of vitamin D status. Thus in our cohort, unlike in previous studies, physical activity was not a risk factor for vitamin D status.
Besides age-related factors that affect the skin and the usual decreased sun exposure that accompanies modern life, skin complexion places elderly at an even higher risk of vitamin D deficiency. Darker pigmentation decreases the skin’s capacity to use sunlight for making vitamin D because melanin competes for UVB radiation effectively [75]. Ethnicity and race are often used as a proxy for skin pigmentation, and a substantial amount of evidence indicates a significant difference in vitamin D deficiency prevalence between different ethnic groups [75]. For example, NHANES 2005-2006 revealed that the prevalence of vitamin D deficiency (25(OH) D<20 ng/ml) was higher in blacks (82.1%), followed by Hispanics (69.2%) and whites (30.9%) [49]. A more recent study evaluated vitamin D status in a community-dwelling elderly population (≥ 60 years old) in South Florida and found higher prevalence of vitamin D insufficiency (25(OH) D<25 ng/ml) among Afro-Caribbeans (30.8%) and African Americans (30.4%) compared to Hispanics (13%) and European Americans (13%) [21]. In our study, we did not find lower levels of vitamin D and thus, higher prevalence of vitamin D insufficiency in African-Americans, as expected, and this could be due to the few African Americans/AfroCaribbeans participating in our study. Only 15.5% of our sample was of African descent while 26% of the population studied by Smolar et al. [21] was either African American or Afro-Caribbean. Nevertheless, we did find a significant association between vitamin D status and ethnicity and a significant difference between vitamin D categories in ethnic groups. We found more Hispanics who had vitamin D insufficiency, while more whiteCaucasians and African-Americans were vitamin D sufficient. When comparing ethnic groups, we found significant differences in vitamin D levels, with Hispanics having lower levels compared individually to whiteCaucasians and African-Americans. In all of our regression models that included ethnicity, we found that this variable had a significant effect on vitamin D levels, with Hispanics expected to have lower vitamin D levels and higher odds of being insufficient. These results also differ from those of Smolar et al. [21], who reported that the prevalence of 25(OH) D insufficiency was not higher in Hispanic participants compared with European Americans. However, their sample population had only 28% Hispanics compared with 43% in our cohort. Thus, ethnicity was the strongest risk factor for vitamin D insufficiency in this study population.
Interestingly, we found a significant difference in height and years lived in the United States, with those who were vitamin D insufficient being shorter and having lived fewer years in the United States. These two factors are related to ethnicity. The average Hispanic person is shorter, and we found a significant difference in height between Hispanics (mean ± SD: 163.29 ± 12.21) and non-Hispanics (mean ± SD:171.12 ± 10.30, P<0.001). Furthermore, Miami being one of the southernmost cities of the U.S, is the home of one of the largest Hispanic communities in the country, which is mostly composed of immigrants. We found a significant difference in years lived in the United Statesbetween Hispanics and non-Hispanics, with Hispanics having lived here for fewer years (mean ± SD:26.63 ± 17.4) compared with non-Hispanics (mean ± SD:59.74 ± 10.66, P<0.001). Immigrant Hispanics may be more likely to be vitamin D deficient and insufficient due to differences in sun exposure practices, public health awareness initiatives in their home countries and less access to healthcare services due to their immigration status.
In summary, the occurrence of vitamin D deficiency and insufficiency in this study was lower than that of other American and European populations. This was expected in a population that has a greater opportunity for vitamin D skin synthesis by year-round sunlight availability. However, vitamin D insufficiency is still a public health concern even in this population. We found that lack of sun exposure, higher %FM, and lack of use of vitamin/mineral supplements were risk factors for vitamin D insufficiency. Ethnicity, however, was the most consistent and important risk factor in this study population, since those who were vitamin D insufficient was more likely to be Hispanic. Since vitamin D deficiency is linked to health risk factors, it is important that health professionals become aware of connections between vitamin D status, intake, bioavailability and skin synthesis to develop dietary and other interventions to prevent and correct deficiency and insufficiency effectively.
This study had several limitations. It was cross-sectional and descriptive in nature and even though this type of investigation can establish associations among factors, it does not prove cause and effect. Second, this study may suffer from selection bias. Besides the expected higher sun exposure of this population, the age criterion could be a possible explanation for the lower occurrence of vitamin D insufficiency observed in our study. The study recruited a younger older adult population (>55 years of age) to have a wider age range and the opportunity to follow them into the older years. According to the World Health Organization, both 60 or 65 years of age are equivalent to retirement age in most developed countries and hence, considered the beginning of old age. In the United States, the age of 65 has been traditionally considered the beginning of senior age [76]. Some institutions like the Florida Department of Elder Affairs, however, considers those >60 years of age as older adults [77]. Furthermore, our age criterion may be a limitation because most studies of vitamin D deficiency and insufficiency in the elderly have been done in adults either above the age of 60, 65, or 70, making our results less comparable. Our study participants were younger than what is considered “old age,” with 36% and 63.8% of participants being under the age of 60 and 65, respectively, and thus, adding heterogeneity to our study. Nevertheless, we did not find a significant difference in age, number of participants over the age of 60, and number of participants over the age of 65, between vitamin D insufficient and sufficient groups. Moreover, age was not a risk factor for vitamin D deficiency in this study population.
The participants in this study were not only younger, but mostly healthier, since criteria for the parent study excluded current diagnoses of numerous diseases/conditions that affect vitamin D metabolism and absorption, medications that disturb vitamin D metabolism, and current vitamin D supplement use. Thus, this sample was not randomly selected from the general older adult population in Miami-Dade, but instead were healthier older adults who volunteered to participate and met inclusion criteria. Thus, the results of this study cannot be extrapolated to the general Miami-Dade older adult population, other populations in the United States or globally.
Vitamin D insufficiency is common in healthy community-dwelling older adults living in Miami-Dade County, especially among Hispanics. Vitamin D intake from food was not a predictor of vitamin D status; however, use of a vitamins/mineral supplement was an independent predictor of vitamin D levels. Factors that affected skin synthesis (ethnicity and sun exposure) and bioavailability/metabolism (obesity) were also significant predictors of vitamin D status. However, ethnicity was the strongest risk factor for vitamin D insufficiency in this study population. Since vitamin D deficiency is linked to health risk factors, it is important that health professionals become aware of the connections of vitamin D status with intake, bioavailability, and skin synthesis and to develop and plan pertinent interventions to prevent and correct deficiency.
JL planned the study, collected, managed and analyzed the data, and wrote the manuscript. AC, FGH, JPL, TL and JEL assisted in planning the study, data analysis and edited manuscript. AHM, SMF, DC, and AMLM assisted in data collection and management and edited manuscript. AF, AR, SEA, ET, JK and JW revised and edited the manuscript.
We are thankful to all of the volunteers who participated in this study. This study was a cross-sectional analysis of the baseline data of the parent interventional study supported by Biotics Research Corporation. Any opinions, findings, and conclusions or recommendations expressed in this material are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
Download Provisional PDF Here
Article Type: Research Article
Citation: Lopez J, Campa A, Huffman FG, Liuzzi JP, Li T, et al. (2016) Correlates of Vitamin D Deficiency in South Florida Older Adults. Int J Endocrinol Metab Disord 2(3): doi http://dx.doi.org/10.16966/2380-548X.127
Copyright: © 2016 Lopez J, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Publication history:
All Sci Forschen Journals are Open Access