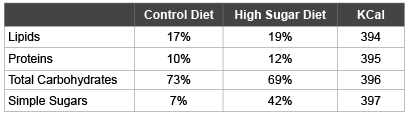
Table 1: Macronutrient composition of experimental diets
Values are represented as percentage of total Kcal.

Marinoni M1 Cordero P1 Martinez JA1,2 Campion J1,2 Gonzalez-Muniesa P1,2*
1Centre for Nutrition Research/Department of Nutrition, Food Science and Physiology, University of Navarra, Pamplona, Spain*Corresponding author: Dr. Pedro González-Muniesa, Centre for Nutrition Research / Department of Nutrition, Food Science and Physiology, University of Navarra, C. Irunlarrea 1, 31008, Pamplona, Spain, Tel: +34 948425600 (806650); Fax: +34 948425740; E-mail: pgonmun@unav.es
Obesity is now considered to be a global epidemic, impacting a great number of women and leading to a higher risk of obstetrical and gestational complications. One of such possible adverse outcomes in gestating female is placental hypoxia, which has been related to vascular remodeling and hypertension, as well as adaptive phenomena to reduce levels of oxidative stress and damage. A pool of female Sprague Dawley rats (n=63) was first assigned into two dietary groups (Control and High Sugar). Following mating, the pregnant rats (n=39) were again distributed into two oxygen treatment groups (Normoxia and Hypoxia) for 3 weeks, and tissue sampling and biochemical analyses were carried out. The main results of this study are the following: 1) Hypoxia during gestation may lead to a reduction in the average number of pups per mother, 2) Hypoxia during gestation treatment may lead to a decrease in maternal serum TG levels, and consequentially 3) Hypoxia during gestation may lead to a reduction in TyG Index levels. These results suggest that hypoxia could generate a beneficial response in pregnant Sprague Dawley rats to salvage both maternal and fetal viability. Thus, reproducing mild hypoxic conditions could result being a viable therapeutic option in preventing gestational adversities. In conclusion, progress was made in recognizing the possible role of a mild hypoxic environment in stimulating a maternal protective response.
Placenta; Fetus; Oxidative stress; Triglycerides; TyG Index; Pregnancy
BMI: Body Mass Index; Group C: Group Control; Group H: Treatment Group “Hypoxia”; Group HH: Group “High Sugar Diet + Hypoxia Treatment”; Group HN: Group “High Sugar Diet + Normoxia Treatment”; Group HS: Diet Group “High Sugar”; HIF: Hypoxia-Inducible Factor; NOS: Nitric Oxide Synthase; TG: Triglyceride; TyG Index: “Triglyceride Glucose” Index; WHO: World Health Organization
Currently, obesity is considered to be a global epidemic [1]. The WHO recorded that around 200 million men and 300 million women have been reported to be in a state of obesity (BMI >30 kg/m2), which in total corresponds to about 10% of the world’s adult population [2]. Furthermore, obesity during pregnancy is becoming ever so common, as well as the incidence of associated obstetrical complications [3]. From a maternal point of view, issues such as preeclampsia or gestational diabetes might happen; from a fetal/neonatal perspective, issues such as macrosomia or deleterious variations in the gestational development could occur [4].
Placental hypoxia is one important obstetrical complication playing a role in vascular remodeling, hypertension, metabolic alterations, oxidative stress, mitochondrial dysfunction, and endoplasmic reticulum stress. All these issues can lead to pregnancy disorders such as preeclampsia or retarded intrauterine growth, among others [5]. During the first trimester of gestation, fetal trophoblastic cells move into the maternal uterine spiral arteries. This is a fundamental process to promote proper fetal irrigation, given that the osmotic pressure in the uteroplacental region greatly depends on the successful migration of these trophoblastic cells, amongst other factors [6]. Furthermore, it has been demonstrated that a failure in proper cellular oxygen sensing is likely to be linked to deregulated placental structure and function often found in pregnancy disorders [7].
In spite of the above mentioned, the placenta has a great reserve capacity that protects the fetus from being affected by a possible hypoxic condition [8]. Nonetheless, the study of placental hypoxic lesions is useful when carrying out a retrospective analysis of possible pregnancy complications [9]. Furthermore, it has been reported that placentas acclimatized to a hypoxic environment (such as in women who lived at high altitudes during gestation) present considerably reduced levels of physiological oxidative stress compared to placentas which had not gone through this acclimatization (such as in women who lived at sea-level) [10]. Interestingly, this situation appears to be due to the fact, that women living at high altitudes present an increased villous vascularization, whereas simultaneously there is a thinning of the villous membranes. These outcomes have a positive impact on the diffusion of oxygen, and the subsequent resistance to hypoxic conditions [11]. In previous animal models, it has been demonstrated how a high-fat diet can lead to an increase in perinatal mortality, as well as a decrease in neonatal survival. This finding can be attributed to an excess of adipose tissue during gestation which stimulates deleterious alterations in the placental vasculature, hence leading to a condition of fetal hypoxia and, consequentially, to an increase of fetal mortality [12].
The purpose of this research project was to study the impact of the exposure to a hypoxic environment, together with a pre-existing state of maternal obesity, on the natural progress of the fetal development. Another focus of interest was to investigate the possible activation of a general maternal protection of the fetuses when exposed to a theoretically deleterious hypoxic environment.
Initially, a pilot group was made up of 6 female Sprague Dawley rats, with an approximate weight of 245 grams, which were provided with an ad libitum “control” diet. Each female rat had its own individual cage. Over the course of 7 days, a pool of male Sprague Dawley rats (n=8) rotated systematically in such a way where every individual male rat spent at least a total of 24 hours with each female rat. This rotation was implemented to ensure successful mating. Once mating was achieved, the progress of the gestation period was monitored and a “weight gain curve” was generated through daily weighing of the female rats.
Later, from a pool of female Sprague Dawley rats (n=63), with an approximate initial weight of 50 g and an age of 5 weeks, 2 groups were formed: Group C was fed the Control diet (n=31), whereas Group HS was fed the “High-Sugar” diet (n=32). Following previously reported guidelines, a diet high in sugar composed of chow and sweetened condensed milk (2:1) was administered to promote a state of obesity in the HS group [13]. The difference in macronutrient composition of the diets is highlighted in Table 1. The diets were maintained throughout the whole study. After 8 weeks of dietary treatment the blood glucose level of every rat was monitored using an Accu-Check Aviva glucose meter (Roche Group, Basel, Switzerland). Also, the quantity of adipose tissue of each rat was determined using an EchoMRI (Echo Medical Systems, Texas, USA).
Female rats were mated with male Sprague Dawley rats (n=8) during a period of 12 days. The weight of every female rat was monitored to determine successful impregnation and to track gestation. Determination of those rats that reached Day 14 of gestation was carried out utilizing a criterion based on the exterior condition of the pregnant rat’s abdomen, as well as the rat’s weight compared with the “weight gain curve” generated from a pilot study.
On Day 14 of gestation, the female rats were randomly allocated to one of the following groups: Group N (normoxia) or Group H (hypoxia). The rats were assigned to a total of 4 subgroups: Control Diet + Normoxia; Control Diet + Hypoxia; HS Diet + Normoxia; HS Diet+ Hypoxia. Due to an unexpected reduction of the number of viable specimen, not related with the treatment, in the Control Hypoxia group (n=3), this group was withdrawn from the study. The Control Diet + Normoxia group will henceforth be referred to as the Control group.
The morning of Day 19 of gestation the female rats were euthanized by decapitation. Day 19 was determined to be safe day to avoid the rats giving birth, while being far enough along gestation to allow an extended analysis of the impact of the diet, as well as that of the oxygen treatment [14]. The night of Day 18 of gestation the rats were given no food in preparation for the sacrifice. The rats were euthanized one at a time utilizing a guillotine. A surgical team of 5 people was responsible for carrying out the sacrifice and subsequent sample collection. All the procedures concerning animals were performed in agreement with the National and Institutional guidelines of the animal care and Use Committee at the University of Navarra (Pamplona, Spain), with the code 052-11.

Table 1: Macronutrient composition of experimental diets
Values are represented as percentage of total Kcal.
Hypoxia treatments were carried out over a period of 5 days. Each rat started treatment with an afternoon session on Day 14 of gestation. The afternoon session of Day 18 of gestation was the last treatment for every rat. Sessions were divided into morning (09:00-14:00) and afternoon (14:30-19:30); 5 hours each. Rats went through treatment once a day, alternating sessions throughout the 5 day period.
The treatment was carried out in closed cages purposely built for the study. The inside of the cages was covered in wire netting in order to avoid the rats accessing the holes made in the sides of the cages to ensure air flow. On the back side of the cage, entry points were made for the tube connected to the gas cylinders. On the inside of the cages was placed a R-17MED Medical Oxygen Sensor (Teledyne Analytical Instruments, Los Angeles, USA) connected to the gas cylinder, passing through a MX300 Medical Oxygen Monitor (Teledyne Analytical Instruments, Los Angeles, USA). The purpose of the oxygen sensor was to interrupt the flow from the cylinder when the levels of oxygen reached levels of ≤ 14%.
The cages for hypoxia and normoxia had the same set up (as explained above), including factors such as temperature and ad libitum water. In the case of the hypoxia cages there was an incoming flow from a nitrogen gas (N2) cylinder, with the purpose of displacing the oxygen within the cages so as to reduce the level of O2 to approximately 14% to generate a mild hypoxia [15]. For the normoxia group, the cages were connected to a pump introducing O2 level of 21% (normal atmospheric level), so as to maintain a similar air flow condition without exposing the rats to hypoxia.
The surgical team was responsible for gathering samples and tissue collection. Glucose and TG serum concentrations were measured in an autoanalyzer Pentra C-200 (HORIBA ABX, Madrid, Spain) with specific kits from this company.
All data are presented as mean ± standard deviation (SD). For the analysis of the impact of the hypoxia treatment on a series of dependent variables a One-Way ANOVA test was carried out, followed by a Tukey Post-hoc test. All data were evaluated using SPSS version 15.0 (IBM, Chicago, USA) software.
A state of obesity was successfully induced in the female Sprague Dawley rats fed the HS diet previously mentioned. After 8 weeks of diet there was a sharp increase in accumulated body fat by the HS specimen compared to those who were fed Control diet. The HS group (HN + HH) registered a mean value of 32,5 ± 1,5 g body fat and 12,2 ± 0,8 % body fat percentage, whereas the Control group recorded a mean value of 27,5 ± 0,8 g and 10,2 ± 0,6 %. Furthermore, the mothers were affected by the hypoxia treatment (Table 2). When compared to both Normoxia groups (C and HN), Hypoxia treatment (HH) appears to lead to a marginal reduction in the mean number of pups per mother (-24%, p<0.1 and -25%, p<0.1, respectively). Instead, comparing only the HS diet groups (HN and HH), Hypoxia treatment (HH) appears to be responsible for a decrease tendency in the levels of maternal serum TG levels (-47%, p<0.1). Finally, Hypoxia treatment (HH), when compared with both Normoxia groups (C and HN), appears to cause a reduction in maternal TyG Index (-5%, p<0.1 and -6%, p<0.05, respectively), with a statistically significant relationship between the HS diet groups (Figure 1). Groups C and HN showed TyG indexes of 9,7 ± 0,4 and 9,8 ± 0,3 respectively, whereas the index of HH group was 9,2 ± 0,3. No statistically significant impact was recorded on other variables.
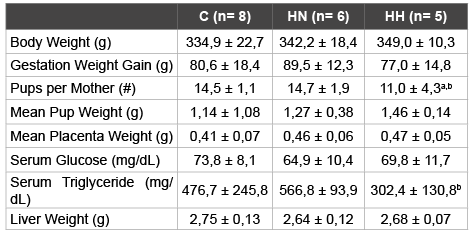
Table 2: Physiological parameters for female Sprague Dawley rats
All data relative to day of sacrifice (n=19). C: Control; HN: HS Diet +
Normoxia Treatment; HH: HS Diet + Hypoxia Treatment.
Statistical significance determined with One-Way ANOVA and Tukey’s test.
a=Statistical tendency with p<0.1 vs. Control; b=Statistical tendency with
p<0.1 vs. HN
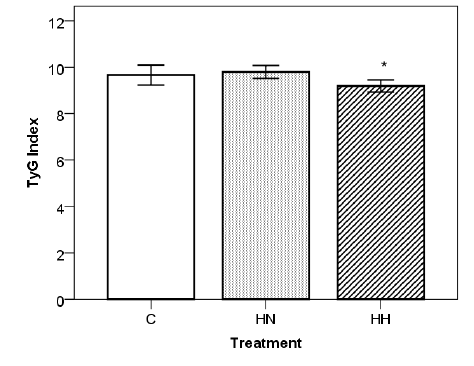
Figure 1: Impact of hypoxia treatment on TyG Index. Statistical significance determined with One-Way ANOVA and Tukey’s test. (*=p<0.05 vs. HN). C: Control; HN: HS Diet + Normoxia Treatment; HH: HS Diet + Hypoxia Treatment. All data (C: n=8 ; HN: n=6 ; HH: n=5) are expressed as mean ± SD.
The negative impact of obesity on maternal and fetal viability has been previously documented [4,12]. Amongst the possible consequences, obstetrical issues for the mother can occur [16], as well as abnormal fetal growth [17] and metabolic impairments for both [18]. One of the hallmarks of the damage caused by a maternal obesogenic condition is the development of placental hypoxia [19], which may induce further complications.
A considerable increase in adipose tissue occurred in the HS group. Considering that the main diagnostic marker when presented with a state of obesity is the quantity of adipose tissue [20], it can be confidently stated that the HS group was afflicted by the condition.
The early stages of gestation are characterized by low oxygen levels [21]. As the placenta develops, it becomes more vulnerable to the damage caused by oxidative stress, in turn generating a defense mechanism dependent on the upregulation of antioxidant genes expression, especially in the labyrinth zone [22]. We described that mothers exposed to hypoxia during pregnancy had lower number of pups, which is similar to previous results found in literature [23]. Given that the treatment started on Day 14 of gestation, the explanation for a reduced litter size cannot be a negative impact on implantation. Interesting for this study is that there is no difference in the mean weight of the pups, hence suggesting that the development of the fetuses was maintained at an optimal level independently of the hypoxia. A possible explanation could be the presence of a mechanism promoting the reduction of litter size as a protective mechanism to sustain maternal and fetal viability [24]. To reinforce this explanation, this study did not find major differences between groups neither in the mean weight of the pups nor in the mean weight of the placentas. It is known that hypoxia triggers a series of systemic, cellular, and metabolic responses that allow tissues to adapt to the damaging effects of the lack of oxygen [25]. Litter size reduction could be one of them, but further investigations are required to identify the mechanisms behind this response.
In general, plasma TG levels often rise during gestation in response to the changes in fuel demands by the mother and fetus [26]. Free fatty acids are taken up and they may be either oxidized as an energy source for the placenta, getting transferred directly to the fetus, or esterified to triglycerides before being delivered to the fetal circulation [27]. From this study, it emerged that hypoxic treatment has an impact on the level of serum triglycerides in obese gestating female Sprague Dawley rats. The decrease in maternal serum triglyceride levels found in the obese mother, after undergoing hypoxia treatment, seems to indicate that hypoxia might be triggering a response mechanism that deters the necessary increase in TG levels. Literature has provided evidence that the condition of obesity may lead the affected individuals to being incapable of successfully removing the excess TG, which in turn play a role in the development of known comorbidities [28]. Furthermore, scientific bibliography indicates that pregnant rats elicit protective mechanisms, such as NOS upregulation, to secure placental development and maintain placental tissue oxygenation when placed in a hypoxic environment [29]. In this context, analyzing the TyG Index – a surrogate marker of insulin resistance – was relevant as it is considered to be a valuable marker of metabolic obesity, given that its values parallel those of metabolic risk parameters [30]. Furthermore, it appears that hypoxia has had an impact in reducing the TyG Index value in the animals fed the HS diet. It is then possible to speculate on the presence of a mechanism intervening to reduce the elevated TG levels when the specimens are set in a hypoxic environment, hence participating in the protective response to the diet-induced obesity.
This study reinforced the need to continue investigating the impact of the relation between obesity and hypoxia on maternal gestation and fetal development. As previously highlighted, hypoxic signals are mediated through HIFs, and an in situ hybridization on placental tissue to determine the presence and distribution of oxygen-modulated HIF-1α and HIF-2α would be highly relevant [29,31]. Furthermore, being able to monitor the development of the fetuses into adulthood would provide a more comprehensive understanding of whether a hypoxic environment can stimulate fetal protection via placental reprogramming. In agreement with the concept of Developmental Origins of Health and Disease (DOHaD), literature suggests that a maternal obesogenic diet could directly lead to long-term consequences on offspring health, including metabolic diseases and increased risk of suffering cardiovascular events [32]. Introducing intermittent mild hypoxia as a treatment to maternal obesity could, by causing an important reduction in maternal TG levels, serve a therapeutic role in preventing the maternal condition having a negative impact on long-term offspring viability.
Exposure to intermittent mild hypoxia, for an overall total of 5 days, leads to a decrease in maternal “TyG Index”. Furthermore, the study highlights two tendencies: exposure to intermittent mild hypoxia, for an overall total of 5 days, leads to 1) a decrease in litter size and 2) a reduction in the levels of serum triglycerides (TG). Overall, progress was made in recognizing the possible therapeutic role of a mild hypoxic environment in stimulating a maternal protective response to adverse conditions.
No potential conflicts of interest relevant to this article were reported. The authors thank Héctor Manuel González Esteban, Paula García Olloquin, Aritz Equísoain Azcona, and Laura Garcia Gerique for excellent technical assistance. The authors also thank Linea Especial (University of Navarra; LE/97) and CIBER obn scheme for experimental financial support.
Download Provisional PDF Here
Article Type: Research Article
Citation: Marinoni M, Cordero P, Martinez JA, Campion J, Gonzalez-Muniesa P (2016) Impact of Hypoxia Exposure, Combined with Induced Maternal Obesity, on Gestating Sprague Dawley Dams. Int J Endocrinol Metab Disord 2(1): doi http://dx.doi. org/10.16966/2380-548X.120
Copyright: © 2016 Marinoni M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Publication history:
All Sci Forschen Journals are Open Access