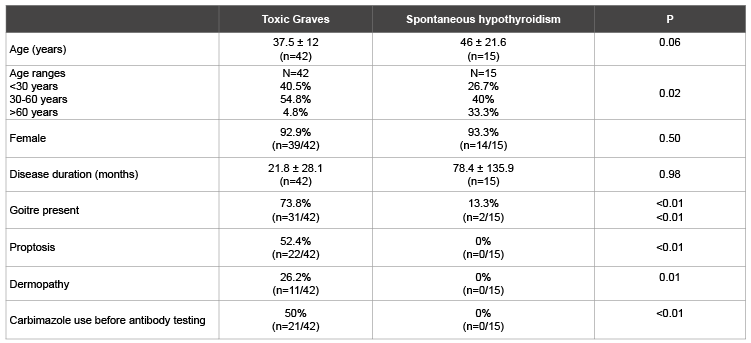
Table 1: Comparison of demographic and clinical profiles of patients with Toxic Graves’ and spontaneous hypothyroidism

Chukwuma O Ekpebegh*1,2 Benjamin Longo-Mbenza1 Ernesto BlancoBlanco3
1Department of Medicine, Faculty of Health Sciences, Walter Sisulu University, Mthatha, South Africa*Corresponding author: Dr C O Ekpebegh, Faculty of Health Sciences, Walter Sisulu University, Private Mail Bag X1, 5117, Mthatha, South Africa, Tel: +27714516355; E-mail: chuksekpebegh@yahoo.com
Background: There are few published reports on the clinical characteristics of auto-immune thyroid disease in the African.
Objectives: To assess the clinical features Thyrotropin Receptor (TR) and Thyroid Peroxidase (TPO) antibodies in Toxic Graves’ and Spontaneous Primary Hypothyroidism (SPH).
Methods: Cross sectional study of black African patients with Toxic Graves’ and SPH attending an Endocrine Clinic in the Eastern Cape province of South Africa over the 5 year-period from March 1, 2008 to February 28, 2013. Data on age, gender, type and duration of thyroid disease, thyroid antibodies were collected. The clinical features and percentages of patients with Toxic Graves’ and SPH who were positive for TR and TPO antibodies were assessed.
Results: There were 42 patients with Toxic Graves and 15 with SPH. Both patients’ groups were dominantly female with ages of 37.5 ± 12 and 46 ± 21.6 years; P=0.06 respectively. The TR and TPO antibodies positivity rates in Toxic Graves’ were 80.5% and 65% respectively. The TR and TPO antibodies rates in SPH were 8.3% and 40% respectively.
Conclusion: Toxic Graves’ in our black African patients is associated with high rate of TR antibody positivity. The low rate of TPO antibodies in our SPH patients may suggest non-immune etiology in the majority of patients with SPH.
Thyroid antibody profiles; Toxic graves diseases; Primary hypothyroidsim
Thyrotropin Receptor (TR) and Thyroid Peroxidase (TPO) are autoantibodies to the TR receptor and thyroid peroxidase enzyme respectively. These antibodies are invaluable in the characterization of thyroid disease as auto-immune and thus useful in the diagnosis of Graves’ disease and Hashimoto thyroiditis[1]. Indeed TR antibody positivity is reported to have 100% sensitivity and 97% specificity for the diagnosis of Graves’ disease [2]. They also predict the subsequent development of thyroid failure [3]. Several studies have assessed thyroid antibody levels in black African populations. These studies were either in persons without thyroid disease or hyperthyroid but of unstated aetiology [4-7]. Only one of these studies tested for TR antibody [7].
Few studies in black Africans have assessed TR and TPO antibodies in thyroid disease of defined aetiology [8-10]. The pattern of TR and TPO antibodies amongst our black patients in the Eastern Cape Province of South Africa which is largely rural is yet to be reported. The study assessed the clinical features and the positivity rates of TR and TPO antibodies, in patients with Toxic Graves and SPH.
This was a cross sectional study of patients with Toxic Graves and SPH who attended the Endocrine clinic of our hospital over the 5 year period from March 1, 2008 to February 28, 2013. After ethical approval was obtained, data on gender, age, presence of goiter, proptosis, dermopathy, type and duration of thyroid disease, TR and TPO antibodies levels were captured.
Toxic Graves’ was diagnosed based on the presence of biochemical primary hyperthyroidism or euthyroidism on carbimazole and any of the following: diffuse non-nodular goitre, proptosis, thyroid bruit, dermopathy, positive TR or TPO antibody. Spontaneous primary hypothyroidism was defined as primary biochemical hypothyroidism or euthyroidism on thyroxine after excluding use of drugs associated with hypothyroidism, radioactive iodine use and thyroidectomy. Excluded were patients with hyperthyroidism from drugs, thyroiditis and gestation, solitary and multiple nodules. Central hyperthyroidism and hypothyroidism were also excluded.
Primary hyperthyroidism was based on serum Thyroid Stimulating Hormone (TSH) below normal with elevated levels of free serum triiodothyronine (FT3) alone or elevated FT3 and raised levels of free serum thyroxine (FT4). Primary hypothyroidism was based on elevated serum TSH and low levels of free serum T4. Our normal reference ranges for TSH, FT3 and FT4 are 0.27-4.2 miu/L, 3.9-6.7 pmol/L and 12-22 pmol/L respectively.
Thyroid antibodies were analyzed at the National Health Laboratory Service. Quantitative determination of TR antibody was by a competitive Electrochemiluminiscence Assay [ECLIA] on a COBAS E170 analyzer [Roche Diagnostics, GmbH, Bhoeringer, Mannheim, Germany]. The detection limits of the assay is 0.3 iu/L to 40 iu/L with analytical imprecision; Coefficient of Variation of 1.9-11.4%. Positive TR antibody referred to serum TR antibody level ≥ 1.75 iu/L.
The TPO antibody was quantitatively determined by a chemiluminiscent assay that was combined with a sequential two-step immune enzymatic assay using the Access Immunoassays System [Beckman coulter, Inc. Brea, California, USA]. The detection limits are 0.35 iu/L to 1000 iu/L with analytical imprecision; Coefficient of Variation <10%. Positive TPO antibody referred to TPO antibody level ≥ 35 iu/L.
Continuous variables were expressed as Mean ± Standard Deviation [SD] while categorical variables were presented as percentages (%). Student t-test and Kruskal-Wallis test were respectively used to compare the means of symmetric and asymmetrically distributed continuous variables. Chi square test was used to compare percentages. Spearmans correlation was performed between TR, TPO antibodies levels and disease duration in Toxic Graves’. The level of statistical significance was P ≤ 0.05. All analyses were performed using Statistical Package for Social Sciences (SPSS) for Windows version 21.0 (SPSS Inc, Chicago, Il, USA).
There were 57 patients (53 females and 4 males) with a mean age of 39.7 ± 15.4 years and age range of 14 to 80 years. There were 42 cases of toxic Graves’ and 15 with SPH. Disease duration was 0 to 12 months in 59.6% (n=34/57), 13 to 24 months in 7% (n=4/57) and more than 24 months in 33.3% (n=19/57) patients. Goiter was present in 57.9% (n=33/57) patients.
Three of the 42 patients classified as toxic Graves’ were negative for both TR and TPO antibodies. Two of these 3 patients were females aged 29 years and 48 years and both had marked bilateral proptosis. The 29 year old patient had thyrotoxicosis for 36 months, with TR and TPO antibodies of 0.9 iu/L and 12 iu/L respectively. She received RAI131 following poor response to carbimazole therapy. The 48 year old was a newly diagnosed thyrotoxic patient with dermopathy but TR and TPO antibodies of 0.9 iu/L and 5 iu/L respectively. The 3rd patient was a 25 year old male with markedly diffusely enlarged goiter and primary hyperthyroidism of 96 months duration. His TR and TPO antibodies were 0.9 iu/L and 18 iu/L respectively. A 63 year old female with a thyroid nodule was diagnosed as Graves’ based on markedly high TPO antibody titer above 100 iu/L though TR antibody negative.
Table 1 shows a predominance of females across both assessed thyroid disease groups. Patients with SPH tended to be older than those with Graves’ disease. About 5% of Toxic Graves’ and 33% of SPH patients were aged above 60 years. Proptosis and dermopathy were documented in half and quarter of Graves’ disease patients respectively. Table 2 shows higher levels of TR and TPO antibodies in Toxic Graves’ than spontaneous hypothyroidism. A higher percentage of Toxic Graves’ than SPH patients were TR antibody positive. There was a tendency to more patients with Toxic Graves’ than SPH being TPO antibody positive.
Table 3 comprises 39 patients with Toxic Graves’ who had results for both TR and TPO antibodies. There were similar percentages of patients with proptosis, goiter and dermopathy regardless of thyroid antibody status. There was a tendency to TR antibody positivity in patients without prior carbimazole treatment compared with carbimazole treated patients.
Figure 1 shows significantly negative correlation between TR antibodies and disease duration; r=-0.55, P<0.01 in Toxic Graves’. Figure 2 shows significant positive correlation between TPO antibody levels and disease duration, r=0.35, P=0.03 in Toxic Graves’.
15 patients with SPH had results for TPO antibody while 12 had TR antibody results. There were 12 hypothyroid patients with results for both TPO and TR antibody; 1 was positive for both antibodies, 4 were positive for only TPO antibodies while 7 were negative for both antibodies. None of these patients had goiter, proptosis or dermopathy. The hypothyroid patient that was positive for both antibodies was a 48 year old female, newly diagnosed with hypothyroidism with markedly elevated TPO antibody of 99 iu/L and mildly elevated TR antibody of 12.63 iu/L.

Table 1: Comparison of demographic and clinical profiles of patients with Toxic Graves’ and spontaneous hypothyroidism

Table 2: Comparison of thyroid antibodies profiles in patients with Toxic Graves’ and spontaneous hypothyroidism

Table 3: Comparison of Toxic Graves’ patients based on antibodies profiles TR: Thyrotrophin Receptor, TPO: Thyroid Peroxidase
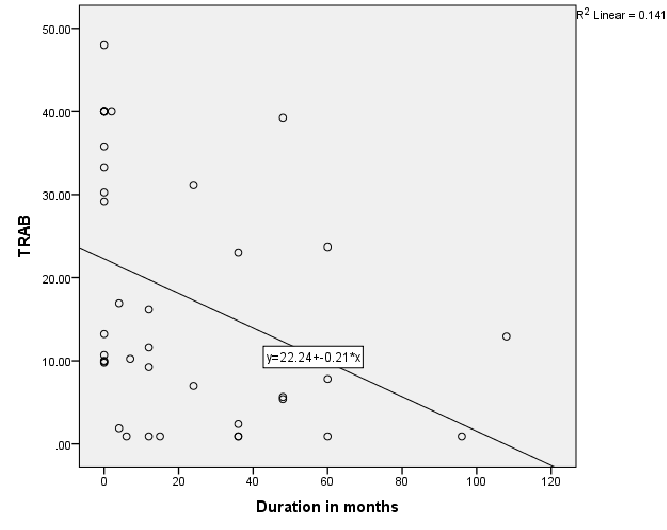
Figure 1: Relationship of Thyrotropin receptor antibody (TRAB) and disease duration in patients with Toxic Graves
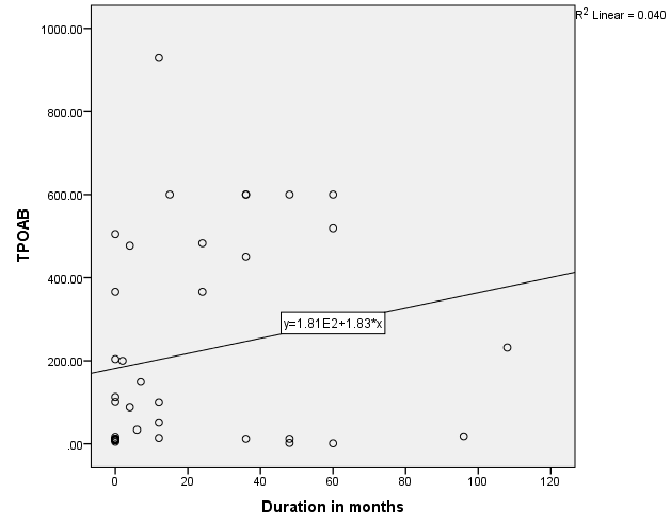
Figure 2: Relationship of Thyroid peroxidase (TPO) antibody and disease duration in patients with Toxic Graves
This study shows Graves’ disease to be rare after the age of 60 years with similar age distribution consistent with another literature report [11]. The TR antibody positivity rate of 80.5% in our Graves’ disease patients is similar to the rate of 83% for black South Africans from a previous study [9], and 90.3% reported in an Asian study [12]. The TPO antibody positivity rate of 65% in our Graves’ disease patients is also comparable to the rate of 66.7% in a Sudanese study [13], that showed the same rates in African and Arab subjects, and the rate of 76.8% in a Nigerian study [10]. The prior use of carbimazole has been reported to attenuate serum thyroid antibody levels [14]. Perhaps TR antibody positivity in our Toxic Graves’ patients may have even been higher if all patients were tested for TR antibody before initiating carbimazole. The negative relationship between TR antibodies and duration of disease in Toxic Graves’ as depicted in figure 1 is consistent with the findings of a study [9], that showed declining antibody titres with longer duration of disease. We however, cannot explain why the same relationship was not demonstrated with TPO antibodies (Figure 2).
This study also shows that about 30% of our Graves’ disease patients had no clinically detectable goiters and only half of our Graves’ disease patients had proptosis which was bilateral in most cases but unilateral in a few patients. Dermopathy was seen in about a quarter of our patients with Graves’ disease and this comprised of symmetrically uniform, nonpitting edema of the feet and legs. We did not observe other described forms of dermopathy [15], namely plaque, nodular and elephantisiatic in any of our Graves’ disease patients. Dermopathy is reported to be strongly associated with opthalmopathy and high TR antibody in Graves’ disease with positive TR antibody in all Graves’ disease patients with dermopathy [16]. The proportions of our Graves’ disease patients with proptosis was 70% (n=7/10) in those with dermopathy compared with 51.7% (n=15/29) in those without dermopathy; P=0.17. We found positive TR antibody rates of 90% (n=9/10) and 75.9% (n=22/29); P=0.20 respectively in our Graves’ patients with and without dermopathy.
One of our patients, a 63 year old female with nodular goiter on palpation and without any clinical auto-immune features of Graves’ disease was classified as Graves’ disease based on markedly elevated TPO antibodies though negative for TR antibodies. A thyroid scan was not done to distinguish a pattern of diffuse uptake seen in Graves’ from solitary or patchy uptake that is expected with Toxic nodule(s). We do appreciate the possibility of misclassification as nodules have been reported in Graves’ and in one study [17], a third of Graves’ disease patients had thyroid nodules.
Thyroid peroxidase antibody has been found to be present in 100% of patients with Hashimotors’ [13], with higher rates of TPO antibody positivity in Hashimotos’ disease than in patients with Graves’ disease [1,13]. The tendency to lower rate of TPO antibody positivity in our patients with SPH in comparison with Toxic Graves’ patients, 40% versus 65%: P =0.06 could indicate non-auto immune aetiology in the majority of our patients with SPH. This may also explain the extremely low rate of TR antibody of 8.3% as only 1 of 12 patients with SPH who had TR antibody result was TR antibody positive. This contrasts with the reported TR antibody positivity rate of 20.4% in Hashimotos’ from another study [12].
The limitations of this study include the small sample size particularly of patients with SPH. We will need to study TPO antibody levels in a larger number of patients with SPH and also perform cytology on the thyroid of patients with spontaneous goitrous hypothyroidism that are TR and TPO antibodies negative to assess for lymphocytic infiltration associated with autoimmune Hashimotos [18].
Toxic Graves’ in our patients is associated with high rate of TR antibody positivity. Spontaneous primary hypothyroidism in our black African population is associated with low rates of TPO and TR antibodies, perhaps suggesting a non-immune aetiology. The pattern of dermopathy in our SPH patients is that of a symmetrical bilateral non-pitting edema of both lower limbs.
Download Provisional PDF Here
Article Type: Research Article
Citation: Ekpebegh CO, Longo-Mbenza B, BlancoBlanco E (2015) Autoimmune Clinical Features and Thyroid Antibody Profiles in Black South Africans with Toxic Graves and Primary Hypothyroidsim. Int J Endocrinol Metab Disord 1(4): doi http://dx.doi. org/10.16966/2380-548X.119
Copyright: © 2015 Ekpebegh CO, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Publication history:
All Sci Forschen Journals are Open Access