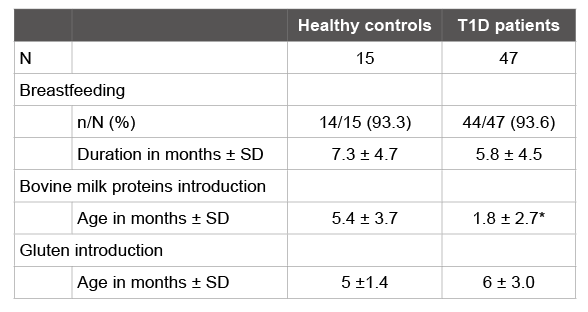
Table 1: Feeding regimes of healthy and T1D children during the first year of life.

María Esther Mejía-León Ana María Calderón de la Barca*
Departamento de Nutrición y Metabolismo, Centro de Investigación en Alimentación y Desarrollo, A.C. Hermosillo, Sonora, México*Corresponding author: Ana M Calderón de la Barca, Departamento de Nutrición y Metabolismo, Centro de Investigación en Alimentación y Desarrollo, A.C. Hermosillo, Sonora, México, 83304, Tel: 52+ 662 2892400 ext. 288; Fax: 52+ 662 2800094; E-mail: amc@ciad.mx
Background: T1D is an autoimmune disorder that has been related to leaky gut, possible due to feeding regimes during the first year of life. IgG reactivity against dietary proteins indirectly assess gut permeability disruption. The aim of this study was to evaluate the association of early feeding regimes with total serum IgG response against related dietary antigens (gliadins, bovine caseins and β-lactoglobulin) in children with T1D at onset and after two years of evolution, and to determine the relative distribution of specific IgG subclasses against these proteins.
Methods: A case-control study with 47 T1D children and 15 healthy controls was performed. Their histories of feeding regimes during the first year of life were recorded and a blood sample was collected at the time of the interview. Total IgG indexes and its subclasses (IgG1-4) were determined by ELISA using monoclonal antibodies.
Results: An earlier introduction of cow’s milk in T1D children was observed as compared to healthy controls. No associations between early diet and the current presence of IgG antibodies were found. However, T1D patients had increased total IgG reactivity to gliadins, caseins and/or β-lactoglobulin. These increases were mainly associated with IgG1, IgG2 and IgG4 subclasses, as observed in other autoimmune diseases with intestinal origin as celiac disease. Gliadins showed the highest potential as antigens in T1D.
Conclusions: The IgG reactivity patterns found in T1D children contributes to understand the effects of leaky gut in T1D and the aberrant immune response associated with intolerances and autoimmunity.
Dietary antigens; IgG subclasses; Leaky gut; Mexico; Type 1 diabetes
Type 1 diabetes (T1D) is one of the most prevalent autoimmune diseases in childhood. It is due to beta cell destruction, usually leading to absolute insulin deficiency. Therefore, their onset is often associated with acute symptoms in association with one or more autoantibodies, and linked to the DQA and DQB genes [1]. Besides genetic predisposition, it is important the interplay of environmental factors, such as the diet, infections and the use of antibiotics; particularly, the early feeding regimes may modify the risk of T1D later in the life. The uses of bovine proteincontaining infant formulas instead of breastfeeding, and the anticipated introduction of cereals in the infants diet, are two strongly associated factors with the autoimmunity processes [2].
Bovine caseins, the main proteins found in infant formula, and β-lactoglobulin, one of the most allergenic bovine milk proteins, have been associated with an increased risk for T1D, regardless of the presence of islet autoantibodies. This is possible due to their structural homologies with the glucose transporter GLUT2 [3] and the retinol binding protein [4], both present in human pancreatic islet cells. Regarding cereals, it has been proposed that wheat gliadins play a role in T1D according to the growing evidence of common risk factors between T1D and celiac disease. A window period has been described, between 4 and 7 months of age, in which wheat introduction to the diet is recommended. Its early or late incorporation has been associated with an increased risk for autoimmunity and T1D [5].
Both gliadins and caseins can alter the permeability of the intestinal epithelium favoring a para-cellular transit [3]. Currently, the “leaky gut” model is one of the most investigated areas, which tries to explain the T1D origin, associating it to other factors such as changes in the gut microbiota composition [6]. However, it is unknown if this model is a common condition in all the T1D patients or if it is modified according to the stage of the disease. Therefore, the evaluation of the humoral response to these antigens may represent an indirect measure to assess intestinal permeability, as a result of their passage through the gut epithelium.
The aim of this study was to evaluate the association of early feeding regimes with total serum IgG response against related dietary antigens (gliadins, bovine caseins and β-lactoglobulin) in children with T1D at onset and after two years of evolution, and to determine the relative distribution of specific IgG subclasses against these proteins.
A case-control study with Mexican pediatric patients from the Children’s Hospital of Sonora State (HIES) and a Pediatric Endocrinology private practice, was conducted. It included 23 T1D children at onset (≤ 2 m of evolution), 24 longstanding T1D children (≥ 2 y of evolution) and a control group integrated by 15 healthy children without T1D or allergy symptoms and with negative total IgE titers. The sample mean age was 9.8 years in the T1D at onset group, 11.2 years in the ≥ 2 year-evolution T1D patients and 9.1 years in healthy children.
An informed consent was obtained from parents and the protocol was authorized by the Ethics Committee of Centro de Investigaciónen Alimentación y Desarrollo, A. C. and the HIES Learning and Research Board. Their histories of feeding regimes during the first year of life were gathered during a semi-structured interview with tutors when they went to their routine appointment with the endocrinologist. Then, a single sample of 3 mL of peripheral blood was collected from each participant, and blood serum was separated and stored at 4°C for further analysis.
A direct enzyme-linked immunosorbent assay (ELISA) was performed to determine the total IgG indexes to gliadins, caseins from bovine milk and β-lactoglubulin (Sigma-Aldrich, USA), according to the method adapted in our laboratory [7]. Briefly, plates were covered with 100 µL of 5 ug/mL gliadins, caseins or β-lactoglobulin in bicarbonate coating buffer (35 mM NaHCO3 , 15 mM Na2 CO3 , 0.05% phenol red, pH 9.6) overnight. After four washes with washing buffer (0.1M Tris/HCl pH 7.4, 0.05% Tween 20, 0.05% phenol red, 15 mM NaN3 ), the plates were incubated for 1 h for blocking with 1% fish gelatin (Sigma-Aldrich) in washing buffer at room temperature. Four more washes were performed and then the plates were incubated 2 h with a 1:50 dilution of patients’ sera in washing buffer. Afterwards four washes were done and bound total IgG was detected with HRP-conjugated anti-human IgG antibodies (Dako, Denmark) in a 1:2000 dilution in washing buffer for 2 h. Following three more washes, a fourth wash was performed with PBS and HRP activity was developed with 3,3´,5,5´ -tetramethylbenzidine. The reaction was stopped with 1 M H2 SO4 . The plates were read at 450 nm (Microplate Reader, Bio-Rad, Hercules, CA).
Absorbance values (optical density) of the sera from healthy children were used to define the cutoff values for each of the tested proteins, considering the mean +2SD. The serum reactivity of IgG anti-gliadins, anti-caseins and anti-β-lactoglobulin was expressed as an index, obtained by dividing the serum absorbance value from each patient by the cutoff values. Index values ≥ 1.0 were considered as positives. Sera with a positive result for total IgG were tested to determine the pattern of reactivity of the IgG subclasses to these proteins in T1D.
To evaluate IgG subclasses, the ELISA method used for total IgG was adapted. Plates previously covered and immobilized with gliadins, caseins or β-lactoglobulin as described above, were incubated overnight with the patients´ sera. Afterwards, four washes were done and bound IgG1, IgG2, IgG3 and IgG4 were detected with each monoclonal mouse anti-human IgG1-4 antibodies (Sigma-Aldrich, USA) in a 1:2000 dilution in washing buffer for 2 h. After four washes, plates were incubated with biotinilated goat anti-mouse IgG antibodies (Southern Biotech, USA) diluted 1:2000 in washing buffer for 2 h. Four more washes were performed and then, incubated for 1 h with 1: 2000 streptavidin-HRP in washing buffer. Finally, after three PBS washes, HRP reaction was developed similarly to the method described above. All measurements were run in duplicate of four dilutions and the reported titers corresponded to the mean values for each sample.
Differences in feeding regimens between groups were evaluated by ANOVA and Tukey-Kramer test and for ELISA results, non-parametric Kruskal-Wallis test were carried out in NCSS-2007. Odds Ratios (95% CI) were calculated to evaluate the associations between feeding regimes during the first year of life and the later total IgG immunoreactivity to gliadins, caseins and β-lactoglobulin. P values under 0.05 were considered statistically significant.
The percentage of children who were breastfed was the same in both healthy and T1D children. However, breastfeeding average duration was 1.5 months lower in the group of children who developed T1D, as shown in Table 1. The introduction of cow’s milk was significantly earlier in patients with T1D, with an average at 1.8 months, while in the healthy children, the average was at 5.4 months. Gluten introduction was between 4 and 7 months, according international recommendations, in all the healthy children from the control group, but not in 53% of T1D patients. Although the means were similar in both groups (p>0.05), the T1D group had greater variability, reflected in a larger standard deviation.

Table 1: Feeding regimes of healthy and T1D children during the first year of life.
The mean values for each group are showed. N: sample size. SD: Standard deviation. Significant differences (p<0.05) between groups are indicated with an asterisk (*).
Several studies have shown associations between age of introduction of bovine milk proteins in infant formulas and the development of autoimmunity and T1D [4] consistent with our results. Recently, Lamb et al. [8] hypothesized that early exposure to bovine milk proteins by feeding with infant formula leads to loss of insulin tolerance and promotes the appearance of insulin-specific T cells. However, their results showed a greater effect in the current intake of milk than the introduction age, mainly in children with low/moderate HLA risk. This is an interesting finding since only 36% of our T1D sample had a high risk genotype, while 64% has low/moderate HLA associated-risk [9].
When the estimated cutoffs values for total IgG were compared against the results of patients with T1D, to calculate their indexes, it was found that 96% of them (45/47) showed a positive reactivity of IgG against gliadins. In addition, 43% (20/47) and 32% (15/47) of T1D patients were also positives for IgG anti-caseins and anti-β-lactoglobulin, respectively. The total IgG positivity proportion against the three proteins was statistically similar in the T1D at onset and long-evolution groups.
No significant associations were found when the relationship between early feeding regimens and IgG reactivity against gliadins, caseins or β-lactoglobulin, were assessed. Thus, for the age of wheat introduction and the presence of high IgG anti-gliadins indexes, an OR 1.63 (0.4-6.1, 95% CI) was obtained. Similar results were for cow’s milk introduction with IgG reactivity against caseins (2.82, 95% CI 0.7-10.8) and ß-lactoglobulin (2.5, 95% CI 0.4-12.3).
Exposure to bovine milk in the first months of life has been associated with elevated titers of IgG subclass antibodies against milk proteins, at least up to 8 years of age. IgG subclasses reach adult levels at different times. IgG1 makes it around 5-7 years, IgG2 around 10 years, IgG3 between 7-9 years, while IgG4 levels tend to increase in the first two years of life and then decline, reaching adult levels until about 13 years [10]. This gradual increase pattern throughout childhood reflects the longterm influence of dietary exposures in the early infancy [11]. Since IgG4 is related to mucosa, it has been investigated in relation to the development of autoimmunity and T1D [11].
Assessing the reactivity of the IgG subclasses against these proteins, in the positive IgG cases, allowed us to find a specific reactivity pattern for T1D, detecting some differences associated with T1D evolution time, as shown in Figure 1. For gliadins, it was found a significant reactivity of IgG1, IgG2 and IgG4 subclasses, being IgG2 related to T1D time of evolution. IgG3 reactivity against caseins was significantly higher in T1D at onset but lower in the longstanding T1D group. Also, for β-lactoglobulin, a similar to gliadins profile was characterized by increased levels of IgG1, IgG2 and IgG4.However, due to its higher immunoreactivity in >95% of the patients, gliadins showed the highest potential as antigen in T1D.
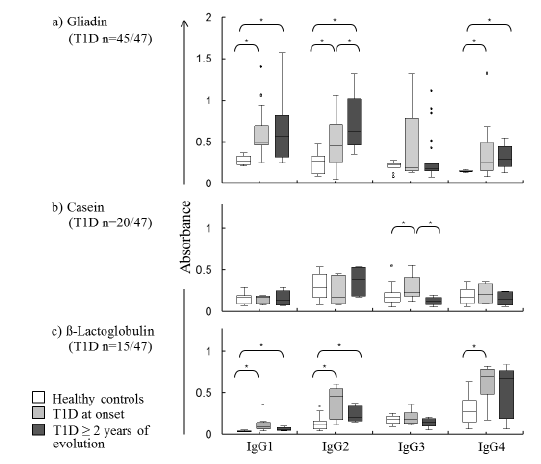
Figure 1: Gliadin, casein and β-lactoglobulin specific IgG subclass antibodies concentrations in children with T1D at onset and ≥ 2 years of evolution compared with healthy controls (n=15). Medians and 25-75 percentile ranges (box) are presented. Significant differences (p<0.05) between groups are indicated with an asterisk (*).
The anti-gliadins and anti β-lactoglobulin immunoreactivity patterns found in children with T1D, characterized by increases in IgG1, IgG2 and IgG4 concentrations, is similar to that reported in adolescents with celiac disease for anti-gliadins [12]. Celiac disease is another autoimmune disorder that shares their genetic and gut origin with T1D. This response cannot be classified exclusively as Th1 (mainly related to IgG1 and IgG2) since both celiac and T1D patients presented higher concentrations of IgG4 subclass, indicative of a Th2 response, which is characteristic of allergic processes. In this regard, IgG4 and IgE production is regulated in parallel; Interleukin-4 from Th2 cells can induce IgG4 and IgE switching in B cells. This may partially explain how diet modulates the proper or inadequate maturation of the immune system in early life.
Together, all these findings support the fact that T1D patients have altered gut permeability, as a consequence of their diet and/or their characteristic inflammatory process. This leads to the passage of dietary proteins across the intestinal epithelium. As a result, it is developed not just a cellular response, but also a humoral, against them. Thus, the inflammatory environment in childhood, proper of pre-T1D and T1D, could be associated with aberrations of the immune system, triggering intolerances or even autoimmunity.
The work had financial support from the Mexican Council for Science and Technology (CONACYT) grant S0008-2009-01-115212. We are thankful to Rene Valenzuela for technical support. The authors declare no conflict of interest.
Download Provisional PDF Here
Article Type: Original Article
Citation: Mejía-León ME, Calderón de la Barca AM (2016) Serum IgG Subclasses against Dietary Antigens in Children with Type 1 Diabetes. J Dia Res Ther 2(1): doi http://dx.doi.org/10.16966/2380-5544.115
Copyright: © 2016 Mejía-León ME, et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Publication history:
All Sci Forschen Journals are Open Access