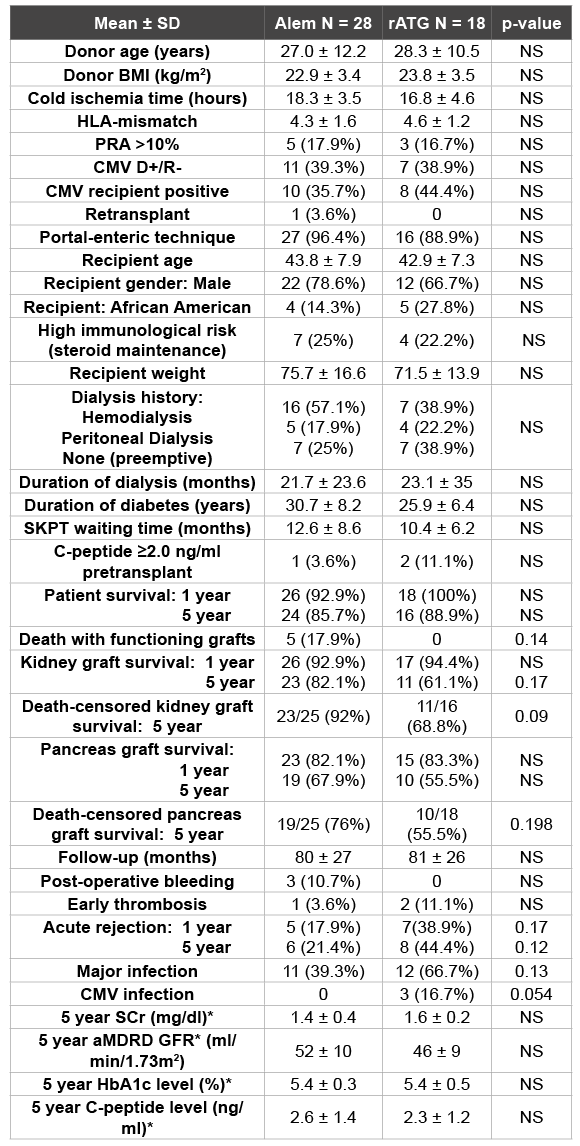
Table 1: Donor and Recipient Characteristics and Results According to Induction Regimen
*In patients with functioning grafts

Jeffrey Rogers1 Alan C Farney1 Giuseppe Orlando1 Samy S Iskandar2 William Doares3 Michael D. Gautreaux2 Scott Kaczmorski3 Amber Reeves-Daniel4 Amudha Palanisamy4 Hany El-Hennawy1 Muhammad Khan1 Jason Bodner1 L Beth Moraitis1 Roberta Brown1 Robert J. Stratta1*
1Department of General Surgery, Wake Forest Baptist Medical Center, Winston-Salem, NC, USA*Corresponding author: Robert J Stratta, MD, Department of General Surgery, Wake Forest Baptist Medical Center, One Medical Center Blvd. Winston Salem, NC 27157, USA, Tel: 001-336- 716-0548; Fax: 001-336-713-5055; E-mail: rstratta@wakehealth.edu
A single center experience with pancreas transplantation (PTx) over an 11+ year period is reviewed.
Methods: We retrospectively studied outcomes in 202 consecutive PTxs in 192 patients at our center. All patients received either rATG or alemtuzumab (Alem) induction with tacrolimus/MMF and tapered steroids or early withdrawal. 179 PTxs (89%) were performed with portal-enteric and 23 with systemic-enteric drainage.
Results: From 11/01 to 3/13, we performed 162 simultaneous kidney-PTxs (SKPT), 35 sequential PTxs after kidney (PAK), and 5 PTx alone (PTA; 40 solitary PTxs [SPT]). 186 PTxs (92%) were primary and 16 pancreas retransplants. With a mean follow-up of 5.5 years, overall patient (87% SKPT versus 87.5% SPT), kidney (74% SKPT versus 82.5% SPT) and pancreas graft survival (both 65%) rates were comparable. Causes of PTx loss were also similar between SKPT and SPT; the rates of early thrombosis were 8.6% and 5%, respectively. Acute rejection rates were similar between groups (SKPT 29% versus SPT 26%, p=NS). A randomized trial of Alem versus rATG induction in SKPT demonstrated lower rates of acute rejection and infection in the Alemgroup; consequently, Alem induction has been used exclusively in all PTxs since 2009. Early steroid elimination has been feasible in most patients. Surveillance PTx biopsy-directed immunosuppression has contributed to equivalent long-term outcomes in SKPT and SPT. Good results have been achieved in African-American patients and in patients with a type 2 diabetes phenotype.
Conclusions: Excellent 5 year outcomes following PTx can be achieved as >86% of patients are alive, >87% of surviving patients are dialysisfree, 80% of surviving patients remain insulin-free, and 88% of surviving patients have detectable C-peptide levels.
Alemtuzumab; Mycophenolate mofetil; Pancreas transplantation; Portal-enteric; Rabbit anti-thymocyte globulin; Simultaneous kidney-pancreas transplantation; Steroid elimination; Surveillance biopsy; Tacrolimus
AA: African-American; Alem: Alemtuzumab; aMDRD: Abbreviated Modification of Diet in Renal Disease; BMI: Body Mass Index; CMV: Cytomegalovirus; DWFG: Death with a Functioning Graft; GFR: Glomerular Filtration Rate; HbA1c:Hemoglobin A1C; HLA: Human Leukocyte Antigen; MMF: Mycophenolate Mofetil; PAK: Pancreas After Kidney; PRA: Panel Reactive Antibody; PTA: Pancreas Transplant Alone; PTx: Pancreas Transplantation; rATG: Rabbit anti-thymocyte Globulin; SKPT: Simultaneous Kidney-pancreas Transplantation; SMV: Superior Mesenteric Vein; SPT: Solitary Pancreas Transplantation; TAC: Tacrolimus; WFBMC: Wake Forest Baptist Medical Center
Vascularized pancreas transplantation (PTx) was initially developed as a means to re-establish endogenous insulin secretion (C-peptide production) responsive to normal feedback controls and has evolved over time to a form of auto-regulating total pancreatic endocrine replacement therapy that reliably achieves a euglycemic state without the need for either exogenous insulin therapy or close glucose monitoring. PTx is performed in patients who require administration of insulin because of type 1 or type 2 diabetes or following total pancreatectomy for benign disease. A successful PTx is currently the only definitive long-term treatment that restores normal glucose homeostasis in patients with complicated diabetes without the risk of severe hypo/hyperglycemia and may prevent, stabilize, or possibly reverse progressive diabetic complications. As of December 2012, more than 42,000 PTxs were reported to the International Pancreas Transplant Registry and Scientific Registry of Transplant Recipients databases [1,2]. PTx in diabetic patients is divided into 3 major categories; those performed simultaneously with a kidney transplant (SKPT), usually from a deceased donor; those performed after a successful kidney (PAK) transplant in which the kidney came from either a living or deceased donor; and PTx alone (PTA) in the complete absence of a kidney transplant. The latter 2 (PAK and PTA) categories are usually combined together as solitary pancreas transplants (SPT) because of similar outcomes and the absence of uremia at the time of transplant. The total number of PTxs steadily increased in the United States until 2004 but has since declined, particularly in the PAK category [1-4]. In the last decade, era analyses of national data have demonstrated that deceased donor recovery rates and additions to the waiting list have decreased; donor organ discard rates and recipient waiting times have increased; and the proportion of recipients who are older, African American (AA), have a higher body mass index (BMI), or are characterized as having type 2 diabetes have all increased [1-4]. The majority (75%) of PTxs are performed as SKPTs whereas approximately 16% are performed as PAK and 9% as PTA transplants [1-4].
With improvements in organ retrieval and preservation technology, refinements in diagnostic and therapeutic technologies, advances in clinical immunosuppression and antimicrobial prophylaxis, and increased experience in donor and recipient selection, success rates for PTx have steadily improved [1-5]. Improvements in outcomes are primarily due to significant reductions in technical failures and immunologic graft losses over time. For recipients of primary deceased donor PTxs, one-year patient survival is more than 95% in all 3 categories; unadjusted five-year patient survival rates are 87% in SKPT, 83% in PAK, and 89% in PTA recipients; and more than 70% of patients are alive at ten years post-transplant [1-4]. One-year PTx survival (insulin-free) rates are 85.5% in SKPT (93% kidney graft survival), 80% in PAK, and 78% in PTA recipients, which translates to pancreas graft half-lives approaching 14 years in SKPT and 10 years in SPT recipients [1-5]. In contrast to other treatments for diabetes, PTx survival is largely defined as complete freedom from exogenous insulin therapy concomitant with the absence of abnormal glycemic excursions. The purpose of this study was to review our single center outcomes and trends in 202 PTxs spanning an 11 year experience.
Indications for PTx were insulin-requiring diabetes with complications and the predicted ability to tolerate the operative procedure and manage the requisite immunosuppression and expected follow-up irrespective of detectable C-peptide levels. Selection criteria for SKPT in type 2 diabetes included patients <55 years of age with a BMI <30 kg/m2 , insulin-requiring for a minimum of 3 years with a total daily insulin requirement <1 u/ kg/day, a fasting C-peptide level <10 ng/ml, absence of severe vascular disease or tobacco abuse, adequate cardiac function, and presence of “complicated” or hyperlabile diabetes [6-8]. Selection criteria for SPT were similar to SKPT except for renal function, in which the calculated abbreviated Modification of Diet in Renal Diseases (aMDRD) glomerular filtration rate (GFR) was >70 ml/min in PTA (native renal function) and >40 ml/min in PAK (renal allograft function) recipients. Donor selection was more stringent for SPT, including younger donors and a minimum of a 2-3 human leukocyte antigen (HLA) match [6,9].
The history of PTx has been largely defined by the evolution in surgical techniques. The first SKPT was performed at Wake Forest Baptist Medical Center (WFBMC) on 6/3/92 [6]. The exocrine secretions were managed with bladder drainage using a donor duodenal segment conduit. Although the patient initially did well with excellent dual allograft function, she ultimately required enteric conversion on 12/20/07 for persistent problems related to bladder drainage including metabolic acidosis, dehydration, recurrent urinary tract infections, and episodes of gross hematuria requiring blood transfusions. The patient’s kidney failed in 2008 secondary to chronic allograft nephropathy and she received a second deceased donor kidney transplant on 1/6/10. However, her original pancreas allograft continues to function well with excellent glycemiccontrol 23 years following transplantation. No other PTxs were performed at our center until November, 2001, and the above pancreas transplant is not included in this analysis.
Since November, 2001, all PTxs were initially approached as an intentto-treat with portal-enteric drainage using an anterior approach to the superior mesenteric vein (SMV) and enteric drainage to the proximal ileum in the recipient (side to side duodeno-enterostomy, usually without a diverting Roux limb) [6,10]. Diverting Roux limbs were used rarely and only if the donor duodenum did not reperfuse well. Arterial inflow was usually based on the recipient’s right common iliac artery after the pancreas dual artery blood supply was reconstructed with a donor common iliac bifurcation “Y” graft. Relative “contraindications” to portal venous drainage were a small SMV (<6 mm in diameter); a deep, buried, or inaccessible SMV (usually associated with central obesity, particularly in recipients with a BMI >30 kg/m2 ); a sclerotic or partially thrombosed SMV or history of venous thrombosis from a previous PTx with portal venous outflow; portal hypertension; or an arterial “Y” graft that would not reach a soft target either on the iliac artery or aorta [10]. In patients (particularly male) with a high BMI, the SMV can be quite deep in the mesentery and the donor common iliac artery bifurcation “Y” graft might not be long enough to reach the recipient’s iliac artery through a window in the distal ileal mesentery, even with the liberal use of a donor artery “extension” graft. In these cases, systemic venous and enteric drainage were performed to simplify the procedure [11].
Of the first 121 SKPTs, all but two were performed by transplanting the kidney to the left iliac vessels and the pancreas to the right common or external iliac artery through a midline intraperitoneal approach. However, since 7/30/10, nearly all SKPTs were performed with ipsilateral placement of the kidney and pancreas to the right iliac vessels in order to reduce operating time and to preserve the left iliac vessels for future transplantation. All but 5 PTxs were performed from brain-dead donors; 5 SKPTs were performed from donation after cardiac death donors at our hospital in which extracorporeal support was used to assist in management of the donor after declaration of death by cardio-circulatory arrest [12].
In SPT and selected SKPT recipients, 2000-3000 units of intravenous heparin (30-50 units/kg) were administered as a single dose during surgery prior to implantation of the pancreas and a heparin infusion was continued post-transplant (continuous infusion of 300 units/hour for 24 hours, then 400 units/hour for 24 hours, and then 500 units/hour until post-operative day 5) in the absence of bleeding [13]. Indications for intravenous heparin included SPT, preemptive SKPT, history of thrombophilia or clotting disorder in the recipient, small or diseased donor or recipient vessels, prolonged pancreas cold ischemia (>15 hours), extended donor criteria, or history of prior pancreas graft thrombosis.
The first 37 patients received alternate day rabbit anti-thymocyte globulin (rATG) induction (1.5 mg/kg/dose, total 3-5 doses) in combination with tacrolimus (TAC), mycophenolate mofetil (MMF), and tapered corticosteroids [14,15]. Subsequently, 5 patients received a single dose (30 mg) of alemtuzumab (Alem) induction intra-operatively, 4 received both single dose Alem and rATG induction, and 16 received rATG induction. During this transitional period, 6 of these patients underwent early steroid elimination. From 2/05 through 10/08, 46 SKPT recipients (45 with portal-enteric drainage) were enrolled in a single center randomized trial comparing single dose Alem (30 mg intra-operatively over 2 hours) and multiple dose rATG (1.5 mg/kg/dose starting intraoperatively) induction in combination with TAC, MMF, and early steroid elimination [16]. rATG induction was administered on an alternate day basis (minimum of 3 doses administered, total cumulative dose 5-6 mg/ kg). TAC was started immediately post-transplant at an oral dose of 1-2 mg twice daily every 12 hours. TAC dosing was titrated to achieve a 12 hour trough level of 10-12 ng/ml for the first 3 months post-transplant, then 8-10 ng/ml thereafter in the absence of rejection or toxicity. Oral MMF was begun immediately after transplant at 500 mg twice daily.
After rATG induction was completed, the MMF dose was increased to 2 gm/day in 2-4 divided doses. The MMF dose was reduced in patients with gastrointestinal intolerance or myelosuppression. After the first 3 months, the usual MMF dose was 1.5 gm/day in the absence of rejection. Corticosteroids were administered either as intravenous methylprednisolone 500 mg or intravenous dexamethasone 100 mg during surgery with subsequent doses given as premedication for rATG. Steroids were completely stopped on post-operative day #5 unless the patient was identified as “high immunological risk” defined by the presence of delayed (kidney) graft function, retransplantation, AA patient <40 years of age, all sensitization (pre-transplant panel reactive antibody (PRA) level >20%), or PTA. Since 2009, all PTx recipients at our center (n=74) have received Alem induction with TAC, MMF, and either early steroid elimination or rapid prednisone taper (dose reduction to 5 mg/day by 2 months following PTx if determined to be high immunological risk) [6,15].
All patients received anti-infective prophylaxis with fluconazole, valganciclovir, and trimethoprim-sulfamethoxazole [6,14,16]. Perioperative antibiotic prophylaxis consisted of a single pre-operative dose, an intra-operative dose, and 2-3 post-operative doses of cefazolin (1 gram intravenous). Patients received single-strength trimethoprimsulfamethoxazole 1 tablet every Monday-Wednesday-Friday for at least 12 months as prophylaxis for Pneumocystis jiroveci. Anti-fungal prophylaxis consisted of oral fluconazole (50-100 mg/day) for 1-2 months. Anti-viral prophylaxis included oral valganciclovir 450 mg/day for 3 months (with dosage adjustments for renal dysfunction and leukopenia) when either the donor or recipient was cytomegalovirus (CMV) seropositive or both were CMV seronegative. If the donor was CMV seropositive and the recipient seronegative (primary CMV exposure), then oral valganciclovir 900 mg/ day (with dosage adjustments as above) was given for 6 months [6,14,16].
Anti-platelet therapy, consisting of oral aspirin (81 mg/day) was administered to all patients. Oral warfarin in a daily dose of 1 mg was administered to patients requiring prolonged vascular access or those with subsequent placement of a tunneled central venous catheter. Most patients were discharged from the hospital after placement of a tunneled central venous catheter and received intravenous fluid and electrolyte supplementation at home for a variable period of time. Treatments of hypertension, hyperlipidemia, anemia, diabetes, and other medical conditions were initiated as indicated, aiming to maintain the blood pressure <140/90 mmHg, fasting serum cholesterol <200 mg/dl, hematocrit >27%, and fasting blood sugar <126 mg/dl.
The diagnosis of renal allograft rejection was suggested by an unexplained rise in serum creatinine level of >0.3 mg/dl or a 25% increase from baseline level and confirmed by ultrasound-guided percutaneous biopsy. Banff criteria were used to determine the grade of rejection [17]. Since March, 2008, all SKPT patients underwent both reperfusion and 1 month surveillance kidney biopsies unless there was a specific contraindication. Banff grade Ia renal rejection episodes were treated with 3 steroid boluses and/or oral prednisone recycle. Banff grade Ia rejection episodes without biochemical evidence of improvement or unresolved infiltrates on a repeat biopsy within 2-4 weeks (persistent or steroidresistant rejection) were treated with rATG rescue therapy. Banff grades Ib, II, and antibody-mediated rejection episodes were initially treated with high-dose steroids and rATG for 5-7 doses depending on biochemical and clinical response. Most patients underwent a 1 month follow-up biopsy after treatment of rejection to document histological improvement. The presence of inflammation either on the 1 month surveillance (subclinical rejection) or follow-up biopsy (persistent rejection) was usually an indication for additional steroid therapy and a subsequent follow-up biopsy.
The diagnosis of pancreas allograft rejection was suggested by an unexplained rise in serum amylase, lipase, or glucose levels and confirmed by ultrasound-guided percutaneous biopsy.
Treatment of rejection was based upon the Maryland Classification System [18], and more recently the Banff 2007 Schema [19], both of which take into account the presence and severity of lymphocytic inflammation, endotheliitis, eosinophilia, acinar or ductal inflammation, and arteritis. Borderline and mild pancreas allograft rejection episodes were treated with steroids whereas all other grades of pancreas rejection were treated with rATG. Follow-up pancreas allograft biopsies were performed to document histological improvement and response to therapy. Biopsies graded as “Indeterminate” were managed by optimizing maintenance immuno-suppression with follow-up biopsies as above. Following SPT, surveillance pancreas biopsies were performed at 3 week intervals [20] until there were 2 consecutive normal biopsies. Clinical biopsies were prompted by biochemical parameters.
Data were compiled from both prospective and retrospective databases, with confirmation by medical record review in accordance with local Institutional Review Board guidelines and approval. Categorical data were summarized as proportions and percentages and continuous data were summarized as means and standard deviations. Univariate analysis was performed by the unpaired t test for continuous variables, the chisquare test for categorical variables, and Fisher’s exact test when data were sparse. Unadjusted actual patient and graft survival rates were reported, and actuarial and death-censored graft survival rates were also determined. Survival curves were computed using the Kaplan-Meier method and compared using the log-rank test. A two-tailed p-value of <0.05 was considered to be significant.
From 11/1/01 through 3/1/13, a total of 202 PTxs were performed in 192 patients, including 162 SKPT, 35 sequential PAK, and 5PTA (40 SPTs). 186 PTxs (92%) were primary and 16 pancreas retransplants (10 of which had their primary PTx performed at our center). All but 4 patients received kidney and PTxs either simultaneously or sequentially (one patient received a kidney following a PTA). In addition, 6 patients (3%) underwent subsequent kidney retransplantation. PTx with portalenteric drainage was performed preferentially; however, systemic-enteric drainage was performed in 23 cases (11%) in which portal-enteric drainage was not deemed possible or safe. Indications for systemic-enteric drainage were pancreas retransplantation (n=9, in which the primary PTx was performed with portal-enteric drainage), central obesity (n=7), and difficult vascular anatomy (n=7). The systemic-enteric drainage group was characterized by more pancreas retransplants (39% versus 4%, p<0.0001), more solitary PTxs (35% versus 18%, p=0.09), more AAs (39% versus 17%, p=0.02) and more patients with C-peptide positive diabetes (30% versus 13%, p=0.054) compared to the portal-enteric drainage group. Although the proportions of male recipients (70% versus 56%), recipients ≥ 80 kg (30% versus 24%), and early relaparotomy rates (48% versus 36%) were all numerically higher in systemic-enteric versus portal-enteric PTxs, respectively, none of these differences were significant. The incidence of early PT thrombosis was 4% in systemic-enteric compared to 8% in portal-enteric PTs (p=NS). With a mean follow-up of 5 years in systemicenteric compared to 6 years in portal-enteric PTx recipients, respective patient survival (70% versus 84%) and pancreas graft survival (61% versus 60%) rates were comparable; respective death-censored kidney graft survival (81% versus 82%) rates were similar.
Of the 16 (8%) pancreas re-transplants, indications for retransplantation were early thrombosis following SKPT (n=9) or PAK (n=1), primary PTx loss secondary to rejection (n=4), primary non-function (n=1), and recurrent auto-immunity (n=1).Types of pancreas retransplants included PTx following SKPT ( n=10), second PAK (n=3), second SKPT (n=2), and second PTA (n=1). Eleven patients underwent allograft pancreatectomy prior to retransplantation and 3 at the time of pancreas retransplantation. There were no instances of early PTx thrombosis in pancreas retransplants compared to an incidence of 8.6% in primary PTxs (p=NS). Six patients underwent kidney retransplantation for either early (thrombosis, n=1) or late (chronic allograft nephropathy, N=5, mean 61 months) graft loss. With a mean follow-up of 72 months in retransplants versus 65 months in primary PTx recipients, respective patient survival (95% versus 86%), kidney graft survival (82% versus 75%), and PTx graft survival (64% versus 65%) rates were comparable.
In the randomized study of Alem versus rATG induction in SKPT, 28 patients (61%) received Alemand 18 (39%) received rATG induction; enrollment in the two groups was not equal because the randomization schema also included patients undergoing kidney transplantation alone. Delayed kidney graft function, PRA>20%, retransplantation, or AAs <40 years of age defined high immunologic risk and determined steroid maintenance (n=11) or early elimination (n=35). Follow-up ranged from 67 to 111 months (mean 80 months). There were no significant differences between the two groups in donor, preservation, recipient, or transplant characteristics (Table 1). There were no significant differences between the two groups in 1-year (93% Alem vs 100% rATG) or 5-year (86% Alem vs 89% rATG) patient survival; 1-year (93% Alem vs 94% rATG) or 5-year (82% Alem vs 61% rATG, p=0.17) kidney graft survival; or 1-year (82% Alem vs 83% rATG) or 5-year (68% Alem vs 56% rATG) pancreas graft survival rates (all p=NS, Figures 1-3 and Table 1). There were slightly more deaths with functioning grafts (DWFG, 18% Alem vs 0 rATG, p=0.14) in patients receiving Alem induction. Consequently, five year death-censored kidney (92% Alem vs 69% rATG, p=0.09) and pancreas (76% Alem vs 56% rATG, p=0.198) graft survival rates were slightly higher in the Alem group (Table 1).
There were no differences in early PTx thromboses (3.6% Alem vs 11% rATG), post-operative bleeding (11% Alem vs 0 rATG), other surgical complications, or readmissions between groups (Table 1). The one-year (18% Alem vs 39% rATG) and 5 year cumulative rates of acute rejection (21% Alem vs 44% rATG, p=0.12) were slightly lower in the Alem group and there was no increased risk of late acute rejection with Alem. In addition, the incidence of major infection (39% Alem vs 67% rATG, p=0.13) was slightly lower in the Alem group. CMV infections were lower (0 Alem vs 17% rATG, p=0.05), and bacterial and fungal infections were slightly lower in the Alem group. In steroid-free patients with 5 year follow-up, 60% remained off prednisone and there was no difference in steroid-free rates according to induction agent (65% Alem vs 54% rATG, p=NS). In patients with functioning grafts, mean serum creatinine levels at 1 year (1.1 Alem vs 1.2 mg/dl rATG) and 5 years (1.4 Alem vs 1.6 mg/ dl rATG), mean calculated aMDRD GFR levels at 1 year (57 Alem vs 55 ml/min/1.73m2 rATG) and 5 years (52 Alem vs 46 ml/min/1.73m2 rATG), mean HbA1c levels at 1 year (5.2% Alem vs 5.1% rATG) and 5 years (both 5.4%), and mean C-peptide levels at 5 years (2.6 Alem vs 2.3 ng/ml rATG, all p=NS, Table 1) were similar in the Alem and rATG groups.
A total of 39 PTxs (36 SKPT, 2 PAK, and 1 PTA) were performed in AA recipients and the remaining 163 in non-AA recipients (161 Caucasian, 1 Asian, and 1 Hispanic; Table 2). The AA group had a longer duration of pretransplant dialysis (mean AA 32 months versus 16 months nonAA, p=0.02), fewer preemptive transplants (5.5% AA versus 28% nonAA, p=0.004), fewer SPTs (8% AA versus 23% non-AA, p=0.04), more PTxs performed with systemic-enteric drainage (23% AA versus 9% nonAA, p=0.02), more patients with a current PRA ≥ 10% (28% AA versus 10% non-AA, p=0.008), more patients with 5-6 HLA mismatches (64% AA versus 42% non-AA, p=0.01), and fewer patients who were CMV seronegative at the time of PTx (28% AA versus 48% non-AA, p=0.03). In addition, the AA group had more patients with a body weight ≥ 80 kg (51% AA versus 24% non-AA, p=0.001), more patients with detectable pretransplant C-peptide levels (≥ 2.0 ng/ml) at the time of PTx (36% AA versus 14% non-AA, p=0.001), and more patients with a shorter duration (≤ 18years) of pretransplant diabetes (38% AA versus 17% nonAA, p=0.004). The latter 3 differences suggested that a type 2 diabetes phenotype was more prevalent in the AA group.

Table 1: Donor and Recipient Characteristics and Results According to Induction Regimen
*In patients with functioning grafts
With a mean follow-up of 5.5 years, overall patient (90% AA versus 86.5% non-AA), kidney (67% AA versus 77% non-AA, p=0.21) and pancreas graft survival (59% AA versus 66% non-AA, p=0.35) rates were comparable (Figures 4-6 and Table 2). The rates of early PTx thrombosis (10% versus 7%) and early relaparotomy (46% versus 36%) were likewise comparable in the AA and non-AA groups, respectively. Cumulative clinical acute rejection rates were similar between groups (33% AA versus 27% non-AA). However, the incidence of death-censored dual graft loss was much higher in AA patients (22% AA versus 6% non-AA, p=0.01). In addition, the death-censored kidney graft survival rate (70% AA versus 87% non-AA, p=0.03) was lower in the AA group. In AA patients who were pretransplant C-peptide positive (n=14) versus C-peptide negative (n=25), there were no differences in mortality (7% versus 12%), kidney graft loss (21% versus 36%), or pancreas graft loss (36% versus 44%) rates, respectively. Based on this analysis, we concluded that PTx in AA recipients was characterized by fewer SPTs and PTxs with portal-enteric drainage, more patients with detectable HLA antibodies and C-peptide levels at the time of PTx, more HLA-mismatching, and more patients with a type 2 diabetes phenotype. Although survival rates and the incidences of early thrombosis and acute rejection were similar, AA patients were at a greater risk for kidney graft loss or dual graft loss compared to nonAA patients in the absence of mortality. This finding may imply either a greater risk for graft loss, better survival in the presence of graft loss, or both, in AA patients.
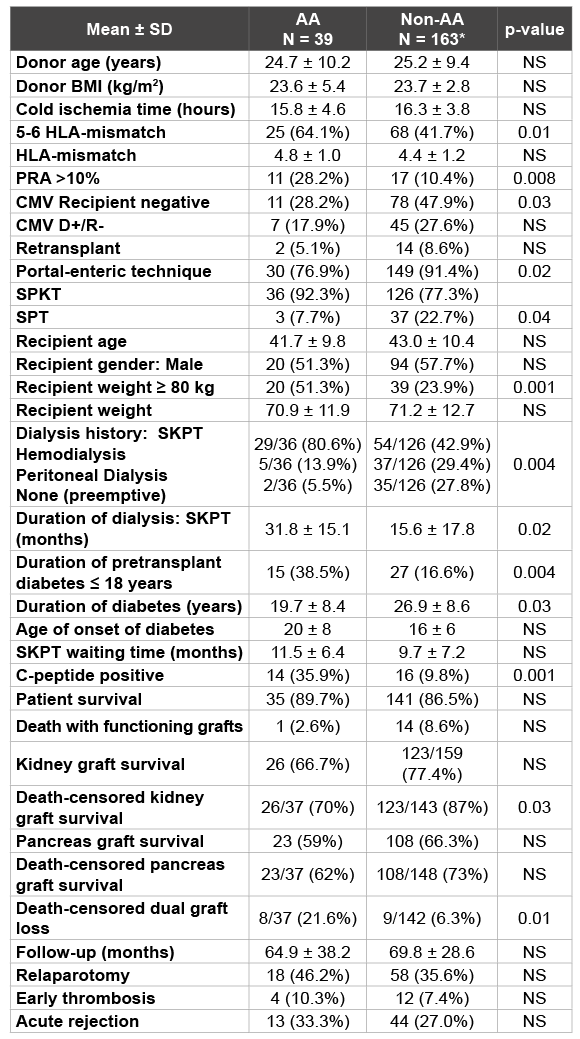
Table 2: Donor and Recipient Characteristics and Results in AA versus non-AA recipients
*161 Caucasian, 1 Asian, 1 Hispanic ethnicity.
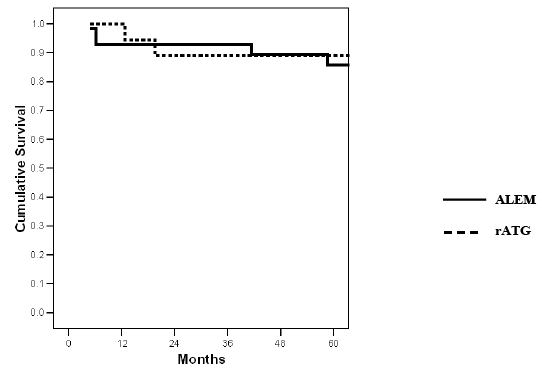
Figure 1: Actuarial patient survival according to method of antibody induction (p=NS).
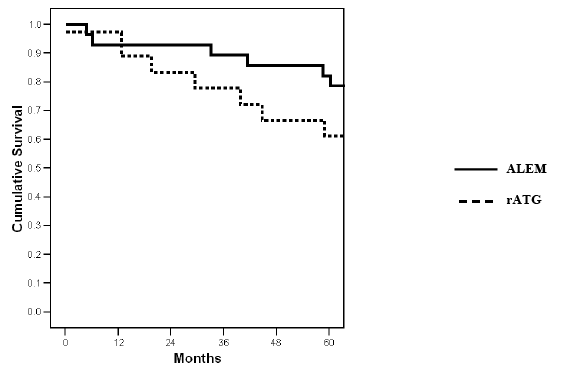
Figure 2: Actuarial kidney graft survival according to method of antibody induction (p=NS).
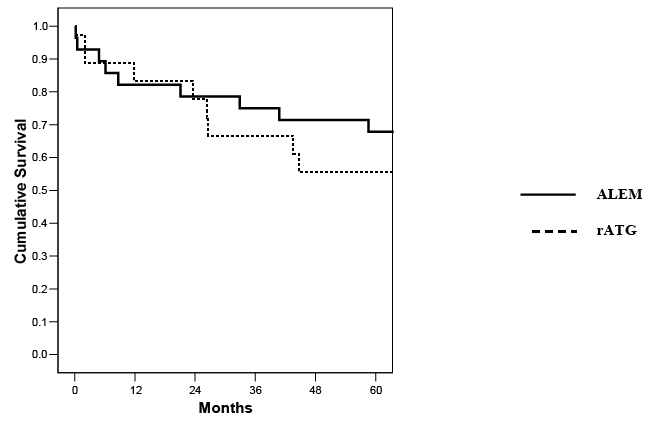
Figure 3: Actuarial pancreas graft survival according to method of antibody induction (p=NS).
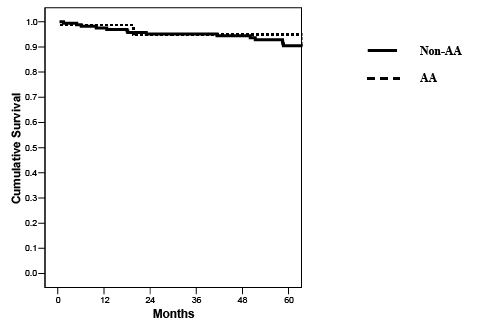
Figure 4: Actuarial patient survival according to recipient ethnicity (p=NS).
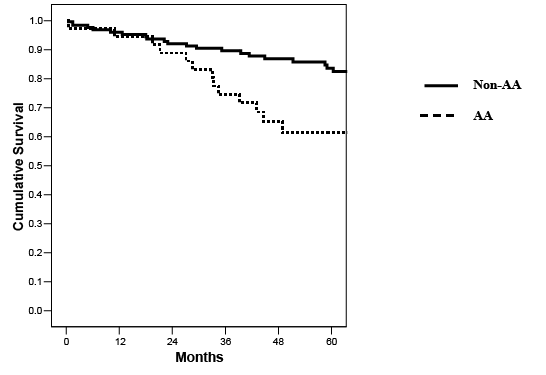
Figure 5: Actuarial kidney graft survival (following SKPT) according to recipient ethnicity (p=NS).
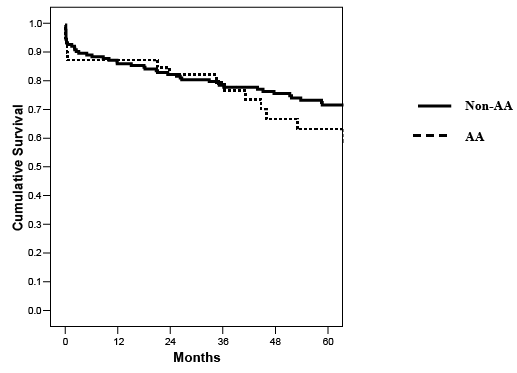
Figure 6: Actuarial pancreas graft survival according to recipient ethnicity (p=NS).
In a sub-study of 26 AA patients receiving either Alem (n=12) or rATG (n=14) induction, mean recipient age (45 years Alem versus 39 rATG), gender (65% male), weight (mean 72 kg), duration of diabetes (mean 20 years), and dialysis duration (mean 27 months) were similar between groups. Pre-transplant hemoglobin A1c (HbA1c) levels (mean 9.3%) were likewise comparable. Detectable pre-transplant C-peptide levels were present in 4 (mean 3.7 ng/ml) and 3 (mean 6.4 ng/ml) patients in the Alem and rATG groups, respectively, suggesting a type 2 diabetes phenotype. With a mean follow-up of 27 months in the Alem and 62 months in the rATG groups, actual patient (100% Alem versus 93% rATG), kidney (92% Alem versus 50% rATG, p=0.036), and pancreas (83% Alem versus 57% rATG, p=0.22) graft survival rates favored the Alem group. Two-year survival rates were all 100% in the Alem compared to 93% patient, 86% kidney, and 79% pancreas graft survival rates in the rATG group (all p=NS). Initial lengths of hospital stay were 9.8 versus 10.4 days (p=NS), but the early re-laparotomy rates were 25% versus 50% (p=0.25), in the Alem versus rATG groups, respectively. The incidence of acute rejection was 16.7% in the Alem compared to 50% (p=0.11) in the rATG group; 5 patients in the former versus 4 in the latter group are currently steroid-free (35% of total cohort). In addition, the incidence of major infection (42% Alem versus 64% rATG) was slightly lower (p=NS). In patients with functioning grafts, most recent serum creatinine (mean 1.2 mg/dl Alem versus 1.6 rATG) and aMDRD GFR (mean 58 ml/min Alem versus 53 rATG) levels were comparable as were most recent C-peptide (mean 3.0 ng/ml Alem versus 4.1 rATG) and HbA1c levels (mean 5.5% in both groups). The most common cause of kidney graft loss was acute rejection (6 of 8 cases) whereas causes of pancreas graft loss included early thrombosis (2), chronic rejection (2), and insulin resistance (4 cases, 3 of which had detectable pre-transplant C-peptide levels). Based on this analysis, we concluded that both Alem and rATG induction were associated with good initial outcomes in AA SKPT recipients, some of whom might be successfully managed with early steroid elimination. Alem induction might be associated with a reduction in early morbidity including less acute rejection and major infection episodes.
We retrospectively analyzed outcomes in SKPT recipients who retained C-peptide production at the time of transplantation and had a type 2 diabetes phenotype. Over an 11+ year period, we performed 162 SKPTs including 132 in patients with absent or low C-peptide levels (<2.0 ng/ ml, C-peptide “negative”, including 21 with measurable C-peptide) and 30 in patients with C-peptide levels ≥ 2.0 ng/ml (C-peptide “positive”, mean C-peptide level 5.7 ng/ml, range 2.1-12.4). At the time of SKPT, patients in the C-peptide positive group had a higher proportion that were age 50 years or older (40% versus 23%, p=0.06), had a later age of onset of diabetes mellitus (mean age 34 C-peptide positive versus 16 years C-peptide negative, p=0.0001), weighed more (mean 77 C-peptide positive versus 69 kg C-peptide negative, p=0.27), and had a greater proportion of AAs (47% C-peptide positive versus 17% C-peptide negative, p=0.001) compared to those in the group with no or low C-peptide levels (Table 3). Pre-transplant duration of diabetes was shorter in the C-peptide positive group (mean 17 years C-peptide positive versus 25 years C-peptide negative, p = 0.01) but duration of dialysis was longer (median 40 months C-peptide positive versus 14 months C-peptide negative, p=0.14). There were no significant differences between the two groups with regard to dialysis status, PRA, HLA-matching, and other pertinent characteristics.
With a mean follow-up of 5.5 years, patient survival (87% C-peptide positive versus 85% C-peptide negative), kidney graft survival (77% C-peptide positive versus 72% C-peptide negative), and pancreas graft survival (57% C-peptide positive versus 66% C-peptide negative, all p=NS) rates were comparable between groups (Figures 7-9 and Table 3). Deathcensored kidney (both 85%) and pancreas (61% C-peptide positive versus 77% C-peptide negative, both p=NS) graft survival rates were similar between groups. The incidence of death-censored dual graft loss was 11% in each group. The 4 deaths in C-peptide positive patients (1 early, 3 late) were due to an early surgical complication, hepatitis C virus infection with cirrhosis, and 2 malignancies, respectively. The incidences of early PTx thrombosis (3% versus 9.8%) and early relaparotomy (33% versus 36%) were no different in C-peptide positive and negative groups, respectively. At 5 years follow-up, there were no differences in acute rejection episodes (30% versus 29%), surgical complications, major infections, readmissions, HbA1c and C-peptide levels, or serum creatinine and calculated MDRD GFR levels between the 2 groups (Table 3). In our experience, diabetic patients with higher C-peptide levels at the time of SKPT appear to have a type 2 diabetes phenotype (older, overweight, more frequently AA, later age of onset and shorter duration of diabetes, and longer duration of pretransplant dialysis) compared to insulinopenic patients undergoing SKPT. However, survival outcomes were similar between groups (Figures 7-9 and Table 3). Consequently, pretransplant C-peptide levels, provided that they are <10 ng/ml, are not used exclusively to determine candidacy for SKPT at our center.
We compared outcomes in 162 SKPT and 40 SPT recipients. All patients were T- and B-cell negative by flow cytometry crossmatch. Demographic characteristics for SKPT versus SPT were mostly comparable (Table 4); however, the SPT group had fewer HLA mismatches (SKPT mean 4.5 ± 1.2 versus SPT2.7 ± 1.5, p<0.001), younger donors (SKPT mean 27 ± 11 versus SPT 22 ± 7.6 years, p=0.004), fewer AA recipients (SKPT 22% versus SPT8%, p=0.03), shorter waiting time (SKPT mean 10 months versus SPT 6 months, p=0.002) but more retransplants (SKPT 1.2% versus SPT 35%, p<0.001). With a mean follow-up of 5.7 versus 7.7years (p=NS), overall patient (86% SKPT versus 87% SPT), kidney (74% SKPT versus 80% SPT) and pancreas graft survival (both 65%) rates were comparable (Table 4 and Figures 10-11). The most common causes of pancreas graft loss were DWFG in SKPT and acute/chronic rejection in SPT recipients. Rates of early thrombosis were 8.6% in SKPT and 5% in SPT patients. The cumulative incidence of clinically evident, biopsy proven kidney or pancreas acute rejection in SKPT was similar to the incidence of clinically evident, biopsy proven pancreas rejection in SPT (SKPT 29% versus SPT 27.5%, p=NS). Based on this experience, we concluded that in the setting of depleting antibody induction, flow cytometry crossmatch testing, HLA matching, careful donor and recipient selection, portal-enteric drainage, peri-operative anti-coagulation, TAC/MMF maintenance immunosuppression, and PTx biopsy monitoring, equivalent outcomes could be achieved in SKPT and SPTs in the new millennium.
Among the 192 patients receiving PTxs at our centerwith a mean follow-up of 5.5 years, mortality was similar (13%) following SKPT or SPT. Mortality rates were likewise similar following primary (13.5%) versus pancreas retransplants (6.25%, p=NS). Although mortality rates were similar, mortality patterns differed as no SPT recipients died early whereas the 1-, 3-, and 5-year mortality rates following SPKT were 4%, 9% and 12%, respectively (p<0.05). In SKPT patients, 15/21 (71%) experienced DWFGs whereas 0/5 patients following SPT (p=0.007, Table 2) had one or both grafts functioning at the time of death (4 had prior kidney graft loss and 3 had prior PTx graft loss). Of the 26 total deaths, 15 were DWFGs, 3 died after kidney graft loss, 6 died after PTx graft loss, and 2 following asynchronous kidney and PTx graft losses. In the absence of either kidney or pancreas retransplantation, mortality rates following isolated kidney, isolated PTx, and kidney-PTx graft losses were 33%, 24%, and 17%, respectively (p=NS). However, 6 patients underwent successful kidney retransplantation and 11 patients underwent successful pancreas retransplantation; mortality following either kidney or pancreas retransplantation or both was 5%. Three SKPT patients died early (within 5 months) of infection secondary to technical issues. Of the remaining 23 deaths that occurred ≥6 months post-PTx (mean 53 months), 11 were cardiovascular, 7 infectious, 2 malignancy, and 3 miscellaneous causes (1 motor vehicle wreck, 1 drug overdose, 1 dialysis withdrawal). The proportion of patients age 50 or older at the time of PTx was higher in those who died (42%) compared to survivors (23%, p=0.05). In summary, mortality rates following SKPT, SPT and pancreas retransplantation were similar, usually occurred late following SPT, and correlated with older recipient age. Following SKPT, the most common mortality pattern was DWFGs whereas mortality following PAK or PTA was heralded by either kidney or PTx graft loss or both. The most common causes of death were cardiovascular and infection regardless of PTx category. Kidney or PTx graft losses were both predictive of subsequent mortality whereas retransplantation of either organ appeared to reduce mortality.
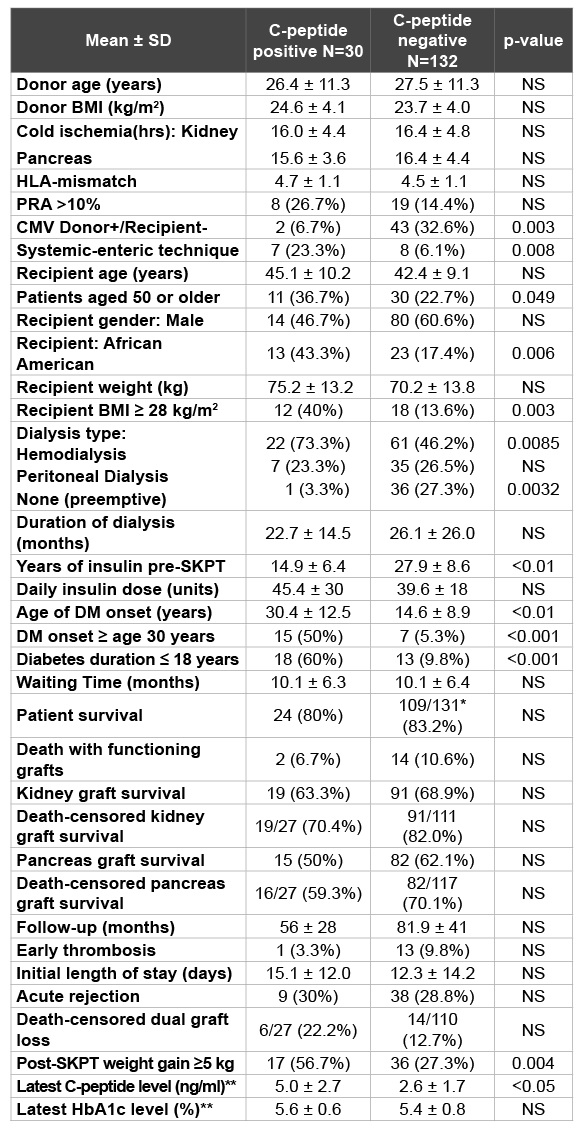
Table 3: Donor and Recipient Characteristics and Results in C-peptide positive versus C-peptide negative SKPT recipients
*One patient who eventually died underwent 2 SKPTs; ** In patients with functioning grafts
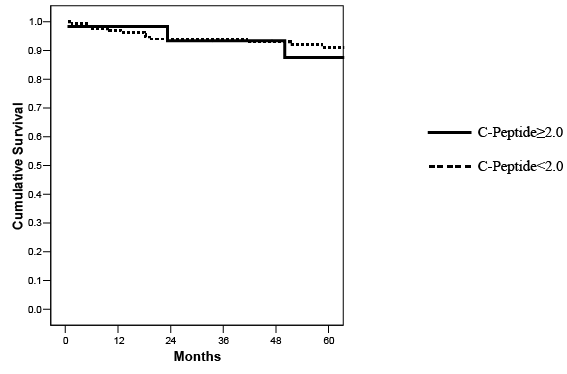
Figure 7: Actuarial patient survival in SKPT recipients stratified according to pretransplant C-peptide levels (p=NS).
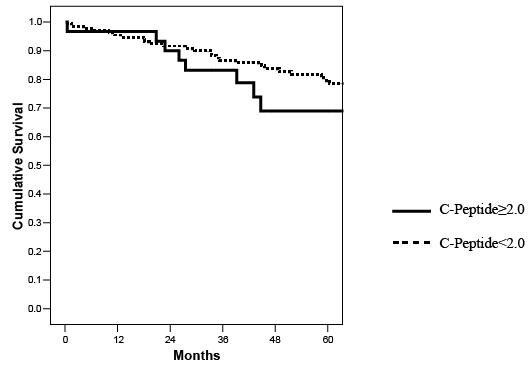
Figure 8: Actuarial kidney graft survival in SKPT recipients stratified according to pretransplant C-peptide levels (p=NS).
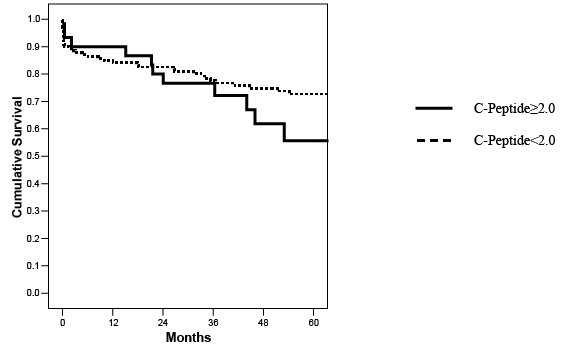
Figure 9: Actuarial pancreas graft survival in SKPT recipients stratified according to pretransplant C-peptide levels (p=NS).
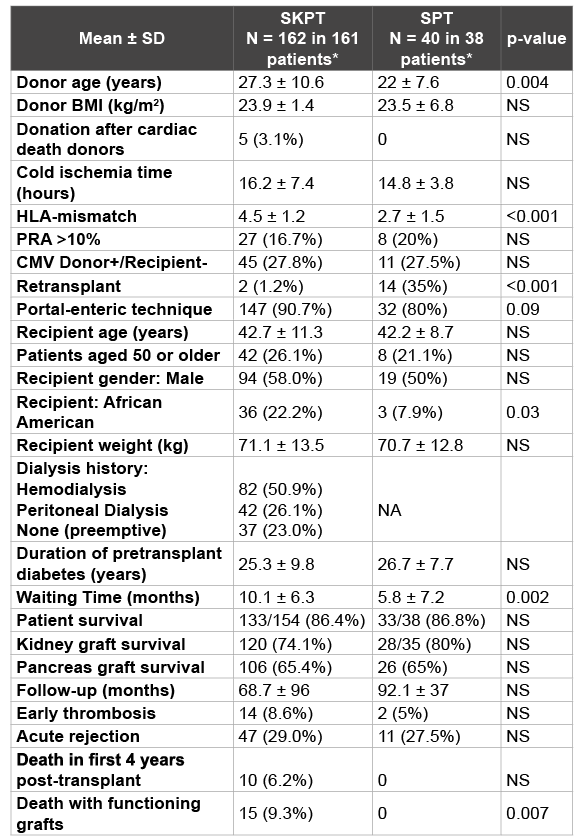
Table 4: Donor and Recipient Characteristics and Results According to PTx category
*One patients had 2 SKPTs, two had 2 SPTs, and seven had SKPT followed by SPTs at our center.
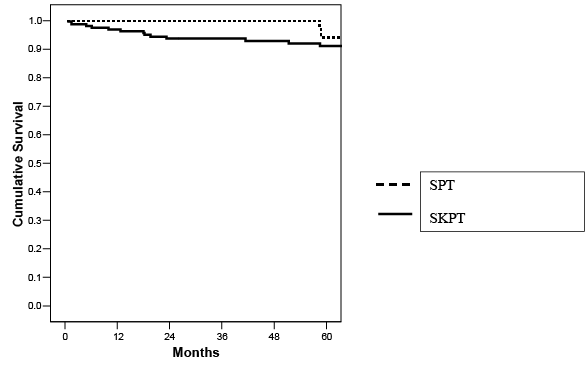
Figure 10: Five year actuarial patient survival according to type of pancreas transplant (p=NS).
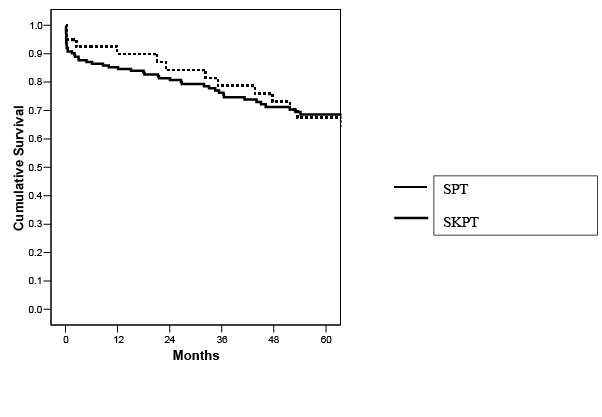
Figure 11: Five year actuarial pancreas graft survival rates according to type of pancreas transplant(p=NS).
Of the 202 PTxs, 70 PTx graft losses occurred, of which 21 (30%) resulted in allograft pancreatectomy. Allograft pancreatectomy was performed in 10% of patients; indications were early thrombosis (n=16), late thrombosis (n=2), rejection (n=1), infection (n=1), and pancreatitis/ uncontrolled leak (n=1). The incidence of allograft pancreatectomy was12.5% in pancreas retransplants compared to 10% in primary PTxs. In addition, the incidence was 13% with systemic-enteric drainage compared to 10% with portal-enteric drainage. With a mean follow-up of 70 months in patients with allograft pancreatectomy compared to 65 months in PTx recipients without allograft pancreatectomy, respective patient survival (81% versus 87%) and kidney graft survival (67% versus 76%) rates were comparable. In summary, allograft pancreatectomy was performed in 30% of PTx graft losses, was usually related to early graft loss secondary to thrombosis, and did not appear to impact medium-term patient or kidney graft survival rates.
The definition of PTx graft failure is not uniform and “success” following PTx may be measured by a number of parameters, including freedom from exogenous insulin and dialysis, absence of hyper/hypoglycemia, enhanced well-being and quality of life, and improved life expectancy. With a mean follow-up of 5.5 years, overall patientsurvival for the entire series (n=192) was 86.5%. A total of 15 patients experienced DWFG whereas 3 patients died following kidney graft failure, 6 following PTx graft failure, and 2 following both kidney and PTx graft failure. Overall kidney graft survival was 75% and the death-censored kidney graft survival rate was 84%. Causes of kidney graft loss (n=49) included DWFG (n=21), chronic allograft nephropathy (n=12), acute/chronic rejection (n=11), polyomavirus nephropathy (n=3), and other (n=2). A total of 6 patients underwent successful kidney retransplantation so the dialysisfree rate in surviving patients was 87.5%. Overall pancreas graft survival (insulin-free rate) was 65% and the death-censored PTx graft survival rate was 72%. Causes of PTx loss (n=70) included early (n=16) or late (>3 months post-PTx, n=3) thrombosis, death with a functioning PTx (n=18), acuteor chronic rejection (n=30), and infection (n=3). A total of 8 patients underwent successful pancreas retransplantation so the insulinfree rate among surviving patients was 80%. In the 30 patients with graft failure from rejection, 4 died, 11 did not have detectable C-peptide, and 15 continued to exhibit C-peptide production and had partial pancreas function although all were insulin-requiring. Using C-peptide production (rather than insulin independence) as the definition of graft survival, the death-censored PTx graft survival rate was 80% and the graft survival rate in surviving patients (including pancreas retransplants) was 88%. Consequently, in patients with severe diabetes, excellent 5 year outcomes following PTx could be achieved as >86% of patients were alive, >87% of surviving patients were dialysis-free, 80% of surviving patients remained insulin-free, and 88% of surviving patients had detectable C-peptide levels.
Our experience with 202 PTxs spanning an 11+ year period is documented herein and chronicles evolving aspects of recipient selection, technical considerations, immunosuppression, and recipient management protocols based on multiple prospective and retrospective studies of our own outcomes. Improving outcomes in vascularized PTx are due to a number of factors including reductions in both technical and immunologic graft losses as well as surgical complications. Compared to SKPT, SPT is associated with higher rates of acute rejection and immunologic pancreas graft loss and lower pancreas graft survival rates even with antibody induction and contemporary immunosuppression (1,3-5). Serum creatinine and urinary amylase levels are not available as markers of rejection in enteric drained SPTs. Additionally, serum amylase and lipase levels are not always reliable indicators of pancreas allograft rejection and do not correlate with rejection grade. Because of the challenges of diagnosing rejection in SPT, we routinely perform surveillance pancreas biopsies in SPT recipients [6,20].
In a retrospective study, Thai et al described excellent short term kidney and pancreas graft survival and a 30% rejection rate using Alem induction and TAC monotherapy [21]. There was no comparison group in this series. Magliocca et al. performed a retrospective review of the University of Wisconsin experience with a 2-dose regimen of Alem induction in SKPT in comparison to historical controls who received basiliximab induction [22]. There were no differences in patient, kidney, and pancreas graft survival rates between groups. Infection rates were comparable between groups except for a significantly higher incidence of CMV infections in the Alem treated patients. This center now uses Alem induction preferentially in SKPT but administers only a single intra-operative dose. Our own study comparing Alem and rATG in SKPT demonstrated lower rejection and infection rates in the Alem group and is unique in that it is one of the few prospective randomized trials of Alem induction in the PTx literature [14,16]. Although our outcomes have prompted us to use Alem induction almost exclusively, we would caution against extrapolating these results to patients receiving other maintenance immunosuppression regimens or to different patient populations.
Our experience with PTx in type 2 diabetic patients compares favorably to other reports in the literature. Nath et al. described the University of Minnesota experience with PTx in type 2 diabetic patients(23).In recipients of technically successful PTxs, 94% were rendered euglycemic in this series. Long-term results were comparable to type 1 diabetic PTx recipients. Light et al reported the Washington Hospital Center 10-year results of SKPT in type 1 and type 2 diabetic patients, defined by the absence or presence of C-peptide, respectively(24). Similar to our experience, the type 2 diabetic patients had a higher BMI, were older at the onset of diabetes, and proportionally had more AA patients. Long-term patient, kidney, and pancreas allograft survival rates were comparable between type 1 and type 2 diabetic recipients in this study. It was concluded that the decision to perform PTx in diabetic kidney transplant recipients should be based on general acceptance criteria rather than diabetes type. Our own data support and confirm this recommendation.
In the recent past, type 2 diabetes was a contraindication to PTx. However, initial intentional (and unintentional) experience with SKPT in patients with type 2 diabetes and end stage renal disease has suggested that augmentation of endogenous insulin production through PTx in patients with C-peptide positive, insulin-requiring diabetes resulted in complete insulin independence, improved glucose counter-regulation, and enhanced quality of life [7]. There may be tremendous overlap in the “definitions” of type 1 versus type 2 diabetes, which are historically differentiated based on age and pattern of onset, detection of C-peptide and islet/antiglutamic acid decarboxylase (GAD-65) antibodies, initial need for insulin and total daily insulin dose, presence or absence of diabetic ketoacidosis, obesity, ethnicity, HLA association, and other associated auto-immune phenomena. To add to the confusion, it is well established that the immunosuppressive medications requisite to transplant may cause type 2 diabetes. Single center and registry reports have documented equivalent SKPT outcomes in patients with either type 1 or type 2 diabetes although clearly a selection bias exists for patients in the latter category [6-8,23,24]. Because SKPT is associated with a shorter waiting time, enhanced donor quality, increased life expectancy, higher graft survival, improved quality of life, and better preservation of renal function compared to deceased donor kidney transplantation alone in diabetic patients, characterization of the “type” of diabetes may be irrelevant and insulin-requiring diabetic patients should be evaluated for SKPT based exclusively on their predicted ability to tolerate the surgical procedure (which has a higher inherent complication rate compared to kidney transplantation alone) and requisite immunosuppression as well as comply with a more stringent post-transplant follow-up regimen compared to kidney transplantation alone. Late immunologic pancreas graft loss continues to be a problem in SPT, even with use of T-cell depleting antibody and contemporary immunosuppression. In our experience, surveillance pancreas biopsies in SPT have revealed a finite incidence of subclinical rejection, allowing for early treatment and optimization of maintenance immunosuppression [6]. We have demonstrated equivalent long-term PTx survival rates in SPT and SKPT and believe that this is related to the early diagnosis and treatment of subclinical rejection episodes in combination with careful graft selection and attention to HLA matching in SPT. The Mayo Clinic experience with surveillance pancreas biopsies has been described by Casey et al. [25]. They observed that minimal grade rejection in SPT rarely progressed to more severe grades of rejection and was not associated with inferior graft survival over a period of 2 years when untreated, although long-term outcomes are unknown with this approach. However, Humar et al. have shown that chronic rejection is the second most common cause of PTx loss after technical failure [26]. On multivariate analysis, the most significant risk factors for PTx loss secondary to chronic rejection were a previous episode of acute rejection and SPT. Based on these data, we continue to favor treatment of early subclinical rejection episodes in SPT although further study and longer-term follow-up is warranted.
At present, 202 PTxs have been performed at WFBMC in the past 11+ years. In the past decade, a number of evolving trends have occurred in PTx at our center including: 1. Conversion from rATG induction with steroid maintenance to Alem induction with early steroid elimination in the setting of TAC/MMF maintenance immunosuppression; 2. Increasing donor and recipient age; 3. Ipsilateral placement of both organs in SKPT; 4. Biopsy-directed immunosuppression, with liberal use of reperfusion, surveillance, clinically indicated and follow-up biopsies both in SKPT and SPT; 5. Successful transplantation of patients with a “type 2 diabetes” phenotype; and 6. A decrease in the annual number of PTxs being performed in the setting of continued growth in our kidney transplant program. The national trend in decreasing numbers of PTxs being performed in the United States is disturbing and probably related to a number of factors including more stringent donor selection (and fewer ideal donors), increasing donor and recipient obesity, overall improvements in the medical management of diabetes (including better insulin analogues, insulin pumps, and sensor devices), financial concerns, and access issues [27,28]. The current impetus to alter pancreas allocation guidelines in the United States based on C-peptide levels or “type” of DM is illogical and not supported by either outcome or utilization data as the overall number of PTxs performed has actually declined in recent years in the absence of the above proposed restrictions.
Vascularized PTx provides an auto-regulating endogenous source of C-peptide that is responsive to normal feedback controls and is currently the only known therapy that reliably establishes a long-term insulinindependent euglycemic state with complete normalization of glycosylated hemoglobin levels. The goals of PTx include freedom from exogenous insulin, better health and well-being, and improved quality of life and life expectancy. Achieving any of these goals might be a reasonable measure of success. For patients with end stage diabetic nephropathy, annual mortality on the waiting list over the past decade has ranged from 7 to 10% [29]. Given the projection that many recipients will live well into their second decade following transplantation, the outcome of diabetic sequelae and rehabilitative potential may be determined by the severity and reversibility of their disease at the time of transplantation. Although PTx results in euglycemia and insulin independence, these benefits are offset by the potential for surgical complications and the short- and longterm sequelae of chronic immunosuppression, leading to a compression of morbidity. SKPT has become accepted as a preferred alternative to kidney alone transplantation in selected recipients with insulin-requiring diabetes because it is associated with superior glycemic control, improved quality of life, enhanced life expectancy, and is cost-effective. In the future, PTx will remain an important option in the treatment of “complicated” insulin-requiring diabetes because of its metabolic efficiency until other strategies are developed that can provide equal glycemic control with less or no immunosuppression or less overall morbidity. Because islet transplant success is defined by C-peptide production and absence of hypoglycemia rather than freedom from insulin therapy and usually involves >1 donor pancreas, future comparisons of PTx versus islet transplant should incorporate similar definitions of graft failure, measures of success, and emphasize longer-term outcomes.
On behalf of all co-authors of the manuscript on hand (A Single Center 11 Year Experience with 202 Pancreas Transplants: Evolving Trends), we assure that the content represents original or unpublished work and has not been submitted for publication elsewhere. We also assure that all authors named on the title page of this manuscript have contributed to the article’s development and have approved the finally submitted version. On behalf of all co-authors, we transfer the exclusive right to publish the manuscript in print and online (in parts or as a whole) to the publisher (the SBDR) and allow publication and storage in third party repositories (such as Medline, PubMed etc.), should the manuscript be accepted for publication. We retain the right to use the results and corresponding patents presented in the article. We assure to refer to The RDS (i.e. the SBDR) as the publisher of the original article in any cases of further utilization. The sender of the manuscript on hand declares upon submission that all co-authors have read and approved this declaration and that they agree that the declaration is effective upon submission to the RDS Editorial Board.
None of the others authors have any conflicts of interest to disclose.
Download Provisional PDF Here
Article Type: Research Article
Citation: Rogers J, Farney AC, Orlando G, Iskandar SS, Doares W, et al. (2015) A Single Center 11 Year Experience with 202 Pancreas Transplants: Evolving Trends. J Diabetes Res Ther 1(3): http:// dx.doi.org/10.16966/2380-5544.111
Copyright: © 2015 Rogers J, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Publication history:
All Sci Forschen Journals are Open Access