
Figure 1: A table from the book [61], commented in the text.
Sergei V Jargin1*
Peoples’ Friendship University of Russia, Clementovski per 6-82, Moscow 115184, Russia*Corresponding author: Sergei V Jargin, Associate Professor, Peoples’ Friendship University of Russia, Clementovski per 6-82, Moscow 115184, Russia, Tel: +7 495 9516788; E-mail: sjargin@mail.ru
Aritcle Type: Mini Review
Citation: Jargin SV (2015) On the RET/PTC3 rearrangements in Chernobyl-related Thyroid Cancer vs. Late Detection. Int J Cancer Res Mol Mech 1(4): doi http://dx.doi.org/10.16966/2381-3318.117
Copyright: © 2015 Jargin SV. This is an openaccess article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Publication history:
Thyroid cancer had been rarely diagnosed in children and adolescents prior to the Chernobyl accident in the areas, where the contamination later occurred, so that there was a pool of neglected cases. Detection by the screening of advanced tumors, interpreted as aggressive radiogenic cancers, coincided with the peak of RET/PTC3 rearrangements. The hypothesis discussed here is that successive waves of thyroid cancer after the Chernobyl accident in people exposed to the fallout at an early age, each wave with different molecular and morphological features, were determined not by radiation but by changing modalities of screening and diagnostics, their improvement with time, and exhaustion by the screening of the pool of advanced cancers. The cohort of papillary thyroid carcinoma in children, diagnosed during the first decade after the Chernobyl accident, where RET/PTC3 was the most prevalent rearrangement type, has been exceptional in the more developed parts of the world: in most series, RET/PTC1 has been the most common type of RET/PTC rearrangement. Considering that the data on sporadic tumors are mainly from more developed countries, this must be caused by the averagely earlier tumor detection. In conclusion, RET/PTC chromosomal rearrangements in papillary thyroid carcinoma RET/PTC3 in particular, probably correlate with disease progression.
Thyroid cancer; RET/PTC rearrangements; Ionizing radiation; Chernobyl
Papillary thyroid carcinoma (PTC), which accounts for about 80% of thyroid malignancies is associated with RET rearrangements [1]. The overall prevalence of RET/PTC rearrangements varies from 20 to 70% of PTCs [2], estimated to be around 28% [3]. The proto-oncogene RET encodes a tyrosine kinase receptor. The oncogene resulting from the protooncogene activation was named RET/PTC after the RET gene and thyroid cancer type PTC [4]. A variety of RET rearrangements are known, but RET/PTC1 and RET/PTC3, involving rearrangement to the H4 and ELE1 genes respectively, are the most common ones [3,5,6]. RET/PTC1 is more common in the classic PTC, its diffuse sclerosing variant and papillary microcarcinoma; RET/PTC3 has been associated with the solid variant of PTC. RET rearrangements are more frequent in the conventional type of PTC than in its follicular variant. According to an estimation, 9% of poorly differentiated thyroid carcinomas harbor RET rearrangements [3], which indicates that a subset of these tumors evolves from PTC with RET rearrangements [1]. The RET locus is virtually never rearranged in follicular or undifferentiated (anaplastic) thyroid carcinomas [1,7].
The RET rearrangements, RET/PTC3 in particular, found in high proportion in PTC of patients exposed to the Chernobyl fallout at an early age [8,9], were discussed as markers of radiogenic thyroid cancer (TC) [10,11]. An association was reported between RET/PTC3 and a less differentiated solid pattern, which prevailed in PTCs of the first postChernobyl decade [5,9]. This time period (1986-1996) is approximately compatible with the meaning of the term “first wave” post-Chernobyl TC, the tumors detected later being regarded as successive waves [5,8,9].
There were many advanced and metastasizing tumors among early post-Chernobyl TCs. In a study from the years 1986-1991, immediately following the accident [12], 61.5% out of 86 histologically confirmed pediatric TC from Belarus were moderately or poorly differentiated; extrathyroidal tumor spread was found in 60.5% and regional lymph node metastases in 74% of the cases. In another study from Belarus (years 1991-92, 84 pediatric TC cases), the tumors were “usually aggressive, often demonstrating intraglandular tumor dissemination (92%), thyroid capsular and adjacent soft tissue invasion (89%), and cervical lymph node metastases (88%)”. PTC was diagnosed in 99% of the cases, with an unusually high frequency of the solid growth pattern [13]. In a study from Ukraine encompassing the years 1986-1996, the most advanced T4 stage was diagnosed in about 50% of all 244 post-Chernobyl pediatric TCs; in adolescents the percentage was even higher: 66-71% [14]. Obviously, a tumor needs time to grow to the T4 stage. High percentage of advanced cases shortly after the accident was obviously caused by the screeningeffect with detection of old neglected cancers, and by the fact that some patients brought from non-contaminated areas were registered as Chernobyl victims. There was a considerable pressure to be registered as Chernobyl victims to get access to benefits and health provisions, which occurred in conditions of uncertainty about doses. The allocation of resources was known to happen not only on the basis of medical need, but rather on the individual’s ability to register as a victim [15]. In cases of advanced tumors it could be seen as a possibility of access to modern therapy. More details are in [16,17].
In the Volume [18], dedicated to the Chernobyl accident, the following statements were made: “The clinical and molecular features of thyroid cancers that developed following Chernobyl are unique” and further: “The prevalence of invasive forms of carcinoma (87.5%) indicates very aggressive tumor development [19]. Clinically this is expressed by a short latency period, absence of general body signs or symptoms, and high lymphatic invasiveness”. Old neglected cases among early post-Chernobyl TCs can explain for these observations [20]; although the “very aggressive tumor development” seems to be an exaggeration, considering that e.g. among 296 (85%) from 348 routinely diagnosed PTCs were reported to be non-encapsulated i.e. invasive [21]. Admittedly, other researchers reported higher percentages of encapsulated TC [22].
RET/PTC3 rearrangement was associated with a more aggressive phenotype, larger tumor size, and more advanced stage at diagnosis [2]. RET/PTC-positive TCs were reported to be associated with the stage III/IV disease, recurrence, and distant metastasis [23]. Local disease recurrence and the development of distant metastases in pediatric patients have also been preferentially associated with RET/PTC3 compared to RET/PTC1 [24]. In the earlier study, no correlation of RET/PTC with a large tumor size, extrathyroidal spread, or metastases was found [7]. However, from the same institution it was later reported that PTC with high RET/PTC1 expression showed an association trend for a larger tumor size [25]. The authors hypothesized that RET/PTC1 may predispose tumor cells to acquire a second RET rearrangement [25], the next frequent being RET/ PTC3 [26], which would mark further disease progression.
The most early TCs after the Chernobyl accident were predominantly solid PTCs with RET/PTC3 rearrangements: 63% of the tumors exhibiting a RET/PTC3 rearrangement were found during first 10 years after the accident, in contrast to RET/PTC1 tumors, which are present in 81% after10 years [9]. The percentage of PTCs with RET rearrangements declined with time, while among RET-positive tumors the percentage with RET/PTC1 increased and those with RET/PTC3–decreased, the RET/ PTC3 rearrangement being the most frequent one during the “first wave” i.e. approximately 10 years after the accident [6,8,9]. RET rearrangements (predominantly RET/PTC3) in Chernobyl-related TC were associated with “increased aggressiveness in terms of pathological stage”, with the most advanced (T4) stage in particular [9,27], the latter being naturally related to the increased aggressiveness in terms of histological grade and disease duration. The first wave TC after Chernobyl had been “significantly less structurally differentiated” than tumors detected later [5]. Detection of advanced TCs, interpreted as aggressive radiogenic cancers, coincided with the peak of RET/PTC3 rearrangements. Accepting the hypothesis that all or majority of the “more aggressive” TCs [8], diagnosed shortly after the accident, were not radiogenic but old neglected cases [16,17], it should be concluded that the RET/PTC3 rearrangements were associated with a later stage of the tumor progression.
As discussed previously [16,28], in the areas, where the contamination later occurred, pediatric TC had been rarely diagnosed prior to the accident. Accordingly, there was a pool of old neglected cancers in the population. The screening started about 3-4 years after the accident, which coincided with the dramatic increase in the registered TC incidence; later on, the pool of undiagnosed TC was gradually exhausted. Besides, as discussed above, some advanced cases were brought from non-contaminated areas and registered as Chernobyl victims. It can be reasonably assumed that accuracy of registration and diagnostic quality were improving with time. This indicates that TCs, diagnosed earlier after the accident, were averagely “older” than those diagnosed later, being at the same time much more advanced than those routinely diagnosed in more developed countries.
The cohort of PTC in children after the Chernobyl accident, where RET/PTC3 was the most prevalent rearrangement type, has been considered exceptional worldwide [29]. In contrast to the Chernobyl data, in most studies dealing with sporadic PTC, the RET/PTC1 has been the most common rearrangement type [6]. It should be commented that the Chernobyl cohort has been exceptional not worldwide but in the more developed parts of the world, where malignancies are diagnosed more efficiently and relatively early [16]. The data from less developed countries are scarce, but they agree with the hypothesis defended here: similarly to Chernobyl, the RET/PTC3 was the most prevalent form of RET rearrangement in a cohort from India [30]. On the contrary, the most frequently observed RET/PTC rearrangement after radiotherapy in France was RET/PTC1 [31], which has been apparently caused by earlier tumor detection in France. TCs “from Japanese children were very different from tumors from countries close to Chernobyl, whether exposed or unexposed, showing significantly more overall differentiation, more papillary differentiation, and less extrathyroid invasion” [32]. A decrease in RET/PTC rearrangements over the last decades in Italy [33] and the USA (along with increasing detection of smaller-sized intrathyroidal TCs) [34] can be explained by the improving diagnostic quality with correspondingly earlier detection of malignancies. Remarkably, no one of the post-Chernobyl PTCs, studied in [35], showed 100 % RETrearranged cells, while in some cases a clustering of rearranged cells was observed. It was hypothesized by the authors that RET rearrangements are late subclonal events [35] i.e. associated with a later stage of the disease progression.
The ability of a screening to enhance the registered TC incidence many times is known [36]. Admittedly, the screening detected not only old neglected cancers but also smaller tumors, latent and dormant TC, microcarcinomas and tumors of uncertain malignant potential, classified as cancers. Besides, histopathological diagnostics was far from perfect during the 1990s; and there was some percentage of false-positivity [17,37]. However, the percentage of earlier lesions must have been increasing with time together with the improving diagnostics and exhaustion by the screening of the pool of old neglected cancers.
It is known that mutations tend to accumulate with tumor progression, and that they can be induced by radiation. RET chromosomal rearrangements were induced by radiation in vitro [24,38-40]; however, the doses used were higher than those after the Chernobyl accident. The report on RET/PTC1 elevation in vitro after an exposure to 0.1 Gy [41] has been exceptional [39] and contradictory to other reports [38,39]. Some statements by Professor Yuri Nikiforov, the last author of [41], directly or indirectly attributing the TC incidence increase “in the U.S. and many other countries” to radioiodine as a result of nuclear explosions or accidents, as well as to medical exposures; and postulating that Chernobyl fallout “resulted in the development of thyroid cancer in more than 4,000 individuals” [43], appear to be indicative of an ideological bias discussed in [28,44,45]: exaggerating medical consequences of the elevated radiation background. There is an opinion that Chernobyl accident has been exploited for the worldwide strangulation of nuclear energy [36]; further discussion is in the Appendix. It should be commented that, as discussed in [16], the incidence of pediatric TC is considerably higher in more developed countries, while the general tendency of the incidence increase is probably at least in part caused by an improvement in the diagnostic quality and coverage of the population by medical checkups.
Correlations between RET/PTC and individual radiation doses were reported [46]; however, such correlations do not necessarily prove causeeffect relationship but can be caused e.g. by dose-dependent differences in diagnostic quality and self-reporting or other factors, discussed in [28]. No association of RET/PTC rearrangements with the dose estimates was found in a study of neoplastic thyroid nodules in children and adults exposed to the Chernobyl fallout in Russia; and the authors reasonably concluded that factors other than radiation could have influenced the development and detection of RET/PTC rearrangements [47]. Finally, absence or very low frequency of RET/PTC rearrangements in follicular and anaplastic TC [1,3,4,7] is probably related to the biological differences between the types of TC i.e. different oncogenic pathways.
Suitability of animal models for conclusions on the RET rearrangements in humans was questioned because of the differences in nuclear architecture between the species [48]. Radiation doses associated with TC induction in rodents were considerably higher than those caused by the Chernobyl fallout. In >6 million residents of the contaminated areas, the average thyroid dose was around 100 mGy, while only for about 0.7% of them the doses were more than 1 Gy [49]. The whole-body doses due to the Chernobyl fallout were much lower. For comparison, 8 Gy of acute whole-body irradiation was used in a recent study in rats [50]. An earlier rat study [51] suggested that the carcinogenic effect from 11 Gy acute x-ray exposure was comparable to a thyroid dose of about 100 Gy from iodine-131, the latter being less carcinogenic by a factor of 10 than an acute dose of x-rays. In adult mice, iodine-131 was one forth to one tenth as effective as x-rays (doses 64-160 Gy from iodine-131 vs. 15 Gy x-ray) for production of TC. At lower doses (22-110 vs. 10 Gy), iodine-131 was found to be one half to one tenth as effective as x-rays [52]. In the study [53], doses from iodine-131 applied in six-week-old rats were 0.8-8.5 Gy, being still considerably higher than Chernobyl doses. The UNSCEAR 1993 Report concluded on the basis of experimental studies that differences in the carcinogenic effect with a factor of about 3 between iodine-131 and x-rays cannot be ruled out [54]. Prior to the Chernobyl accident, carcinogenicity of iodine-131, used in humans for diagnostic and therapeutic purposes, was generally regarded to be low or nil; references are in [54,55]. The number of TCs diagnosed among persons exposed in childhood after the Chernobyl accident was considerably higher than that expected on the basis of preceding knowledge; such a high incidence and short induction period had not been experienced in other exposed populations [56]. Considering the wide variation of individual thyroid doses, development of radiogenic TC after the Chernobyl accident cannot be excluded, but, in the author’s opinion, their supposed numbers have been largely overestimated [16,17]. Reliable data on dose-effect relationships can be obtained in large-scale animal experiments.
In conclusion, the RET/PTC rearrangements in PTC after the Chernobyl accident, the RET/PTC3 in particular, were probably related to the disease duration and a correspondingly later stage of tumor progression; so that the “successive waves of tumors in those exposed to high levels of fallout as children, each with different molecular, morphological, and clinical findings” [8] after the accident were largely determined by evolving modalities of screening and diagnostics, their improvement with time, and exhaustion by the screening of the pool of old neglected cancers. Further research should test correlations between expression levels of different types of RET rearrangements and tumor size, disease duration, histological grade of PTC, axillary lymph node metastases, and patient survival. Data on RET/PTC1 and RET/PTC3 prevalence in routinely diagnosed TC from less developed countries [30] compared to more developed parts of the world may provide further evidence in support of the hypothesis defended here.
The paper [57] was discussed in [58] under the subheading “The Nuclear Establishment is Running out of Dirty Tricks”, although no particular tricks were described. However, tricks such as misquoting and manipulations with statistics, obviously aimed at exaggeration of Chernobyl consequences, were discussed previously [57,59,60]. Here follow some more examples.
With regard to the Volume [12], not much can be added to the previously published examples of incorrect citation etc. [56,58], left without comment in [57]. It should be added that the first author of the volume [18] applied misquoting also earlier. Figure 1 is a photograph from the book [61]. Translation of the bottom table: “Equivalent dose rate 50 mSv/year. Consequences of the exposure: decrease in the life duration of an ‘average human’ by 15 months after irradiation during 5 years (Grahn et al. [62] after Moskalev, Streltsova, 1978 [63])”. The article [62] is quoted second-hand, from a Russian publication [63]. Figure 2 is a copy from [63]. Translation of the bottom paragraph: “According to the calculations by D. Grahn, G.A. Sacher, R.A. Lea et al. [62] the radiation dose of 2, 5 Sv (i.e. 0,05 Sv yearly during 50 years of work, which today is the upper limit for occupational workers) received as chronic irradiation during a very long time, can cause reduction of life duration by 15 days for an average mouse aged 100 days and by approximately 15 months for an average human aged 20 years” [63]. This passage was misquoted in [61], while the key figure 50 was changed to 5, which created a wrong impression about complications of exposure to low dose low rate radiation. Figure 3 represents another example from the same book [61]. The table contains information about medical consequences of Chernobyl accident. Translation of the second line of the table (emphasis added): “Leukemia in infants 1987-1988. Increase in the number of cases in the contaminated territories: Wales and Scotland 3.3 times; Greece (0.2 mSv) 2.6 times; USA (10 mkSv) by 30% (Gibson et al., 1988 etc.) [64]”. Figure 4 represents a copy of the table from [64]. The statement about 3.3-time incidence increase of childhood leukemia in Wales and Scotland in the years 1987-88 can be related only to the first line of the table containing the following figures: 1971-80 (15 cases); 1981-86 (9 cases) and the year 1987 (6 cases) [64]. In [64], the figures were not discussed in connection with the Chernobyl accident, one year being obviously too short period for initiation of radiation-induced malignancy in humans. These examples demonstrate how inaccurate citation can create exaggerated impression about Chernobyl consequences.

Figure 1: A table from the book [61], commented in the text.
Probably the best-known publication by the same author was the socalled “Yablokov Report” [65] on the radioactive waste disposal in seas adjacent to Russian territory. The article has no references and no clear conclusions, the most significant figure being 325 kCi, which is the estimated total activity of the wastes dumped in seas adjacent to Russian territory (contamination due to nuclear testing was not discussed). A supposed maximum of this value was discussed to be as high as 2,500 kCi. Only the latter figure, not corroborated in the text of the article, is given in the open access abstract [65], which is obviously misleading. Doubtful studies [66-68] with participation of other co-authors of the volume [18] were commented elsewhere [57,69].
It is known that among the causes of the Chernobyl accident was disrespect for written instructions [70]. A possible deviation from construction technology of a reactor foundation at the Kola Nuclear Power Plant, reported to the Construction Management, was discussed previously [28]. The only photograph of the foundation pit in possession of the author was submitted for publication in 2004 with a manuscript to the publishing house Meditsina (editor-in-chief–Professor Andrei Stochik); the manuscript was not published and the photographs have not been returned to the author in spite of repeated requests (Figures 5 and 6). After the accident, numerous poorly substantiated publications appeared, where spontaneous diseases in exposed populations were a priori considered to be radiogenic (some cited in [18], discussed in [55]). Unreliability of some later more skillfully formulated reports can be assumed by analogy, because motivations remained unchanged. For an inside observer it is evident that behind the avalanche of papers overestimating Chernobyl consequences was a directive, which has been not unusual for the Soviet science. The Chernobyl accident has been exploited to strangle the worldwide development of atomic energy [36].

Figure 2: Page 32 from [63].

Figure 5: A letter from the author asking to return photographs submitted for publication with a manuscript. Handwritten at the bottom: “The letter of 03.11.04 not received”.

Figure 3: A table from [61].
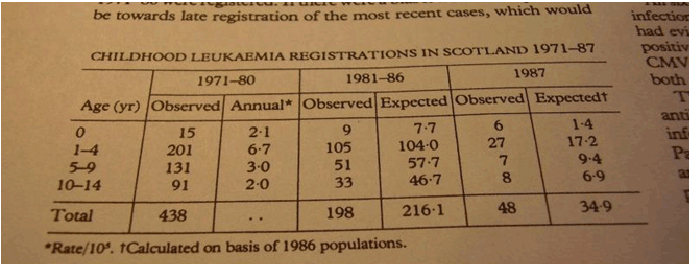
Figure 4: A table from [64].

Figure 6: Postal receipt of 04.11.04 (the letter was dated 03.11.04) confirming that 14 photographs were sent to the publishing house Meditisina together with other materials; the photographs have not been returned to the author.
Download Provisional pdf here
All Sci Forschen Journals are Open Access