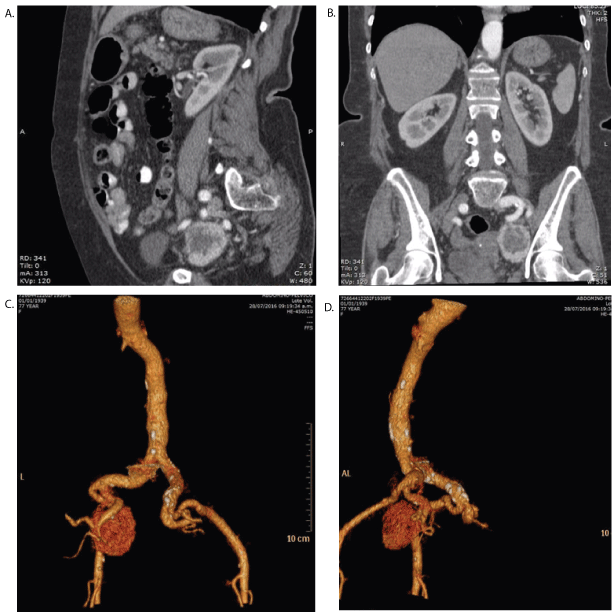
Figure 1: A & B-Contrasted CT scan showing a left hypogastric tumor, contrast enhanced, heterogeneous and diameter of 45 × 34 mm. C & D-CT scan angiography shows left and posterior tumor, highly vascularized depending of hypogastric artery.
Héctor Bizueto-Rosas1* Hugo Alonso Pérez-González2 Carlos Daniel GómezCalvo1 Luisa Fernanda Hernández-Rivera1 Rafael Aburto-Pérez1 Rafael ArmentaLópez1 Javier Ismael Hernández-Vázquez11 Gloria Selene López-Arce1 Carla Isabel Moreno-Ramírez1 Rodrigo Marcelo Maitret-Velázquez1 Perla Elín Leyva-Rivera1 Nayely Leticia Jiménez-Tejeda1 Martha Carolina Rosales-Ramos1 Delio Felipe Martínez-Blanco1 Noemí Antonia Hernández- Pérez3
1Vascular Surgery and Angiology Department at Specialties Hospital, “La Raza” National Medical Center, Mexican Institute of Social Security, Mexico City, Mexico*Corresponding author: Héctor Bizueto Rosas, Puerto Zihuatanejo 18, Colonia Ampliación Fernando Casas Alemán, Ciudad de México, Tel: +52 (55) 7228 8580; E-mail: dr_bizueto_h@yahoo.com
Objective: We report the case of a 77-yeard old female with history of urinary bladder paraganglioma that during follow-up was diagnosed with a tumor in the left hypogastric artery.
Introduction: Paragangliomas are tumors that can be found in the cervical, thoracic, and abdominal spaces. Distal locations surrounding arterial structures such as the aortic bifurcation or iliac arteries are rare, as is the involvement of other pelvic organs like the urinary bladder. Iliac artery and urinary bladder paragangliomas can be considered as paragangliomatosis, or metastasis of each other. The preferred study to locate the non-adrenal paraganglioma is Magnetic Resonance Imaging (MRI). Stage T2 has an excellent definition of size, vascular relationship and metastasis location, being sensible up to 100%. Scintigraphy using I-131-metaiodobenzylguanidine (I-MIBG) has a sensibility of 95% and specificity of 100%, useful at metastasis detection. Surgical resection is the preferred treatment. Pre-operative preparation involving imaging study and pre-medication is vital to avoid hypertensive crisis or vascular collapse due to ceased catecholamine production, as most tumors are functional.
Discussion: Being a rare disease there is no standard surgical approach; partial or radical cystectomy, with lymphadenectomy, is recommended depending on size, location and surrounding tissue involvement.
Conclusion: Determination of catecholamine levels is vital in asymptomatic patients. Levels of metanephrines serum is the most sensitive and specific test. Genetic study in multiple paraganglioma is mandatory. MRI is the preferred location study.
Urinary bladder paraganglioma; Iliac paraganglioma
We present a 77-year old female, born in Mexico City, with no relevant family history. The patient was smoker for more than 50 years, 2 to 3 cigarettes per day. During November of 2014, she presented symptoms of macroscopic haematuria, diffuse abdominal pain and occasional blood hypertension. An initial urinary bladder ultrasound showed a “vascularized tumor, deep into the left wall, measuring 32 × 24 ×27 cm with Doppler color enhancement, and a calculated volume of 10.8 cc”. The patient underwent a transurethral urinary bladder tumor resection, with the obtained tumor being sent for a definitive histopathological study.
Tumor analysis concluded it was a “paraganglioma involving urinary bladder muscular layer”. Slides obtained were revised with immunoperoxidase stain (410-15), chromogranin stain, synaptophysin and S100 protein being positives and CK AE1 /AE3 being negative, making diagnosis of the aforementioned paraganglioma. Metanephrine determination in 24 h urine was performed with results of Total metanephrine 337 µg (<900 µg), normetanephrine 255 µg (<600 µg), and metanephrine of 82 µg (<300 µg) all within reference parameters.
During follow-up in 2016, the patient was sent to our Vascular Surgery Department after a contrasted CT scan revealed a pelvic tumor involving iliac vessels. We performed a specific CT scan with abdominal and pelvic angiography that showed a heterogeneous tumor, contrast enhanced, of 45 × 34 mm diameter, at the left hypogastric artery with adhesion to the surrounding vein structure (Figure 1). Patient reported no specific symptoms during or after contrasted study. Tumor was found to be not functional by the Endocrinology Department.

Figure 1: A & B-Contrasted CT scan showing a left hypogastric tumor, contrast enhanced, heterogeneous and diameter of 45 × 34 mm. C & D-CT scan angiography shows left and posterior tumor, highly vascularized depending of hypogastric artery.
After preoperative planning, surgical resection was performed in December 2016, with a transperitoneal approach, having vascular control of the left common iliac artery, left external iliac artery and proximal and distal segments of the left hypogastric artery; resection was performed by dissection of the tumor and its limits, without damaging the hypogastric artery with a total bleeding of 1100 ml.
Post-surgery ileus was managed with a conservative approach, and was discharged after 7 days in good condition. In the following months, the patient presented an abdominal wall hematoma with local infection, which was managed with antibiotic therapy and wound care, having an excellent recovery afterwards.
Histopathological study concluded “left hypogastric artery paraganglioma of 4 × 3.5 × 3 cm, with 3% necrosis, 10 × 10 mitosis in field, atypical cellular areas with marked pleomorphism that focally infiltrates capsule and surrounding fat tissue. Lesion is within surgical limits. A lymph node of 0.5 cm with paracortical hyperplasia was found”.
Conclusion of Paragangliomatosis with high probability of malignant iliac paraganglioma due to multiple mitosis within surgical limits versus metastasis of urinary bladder primary tumor (Figure 2).
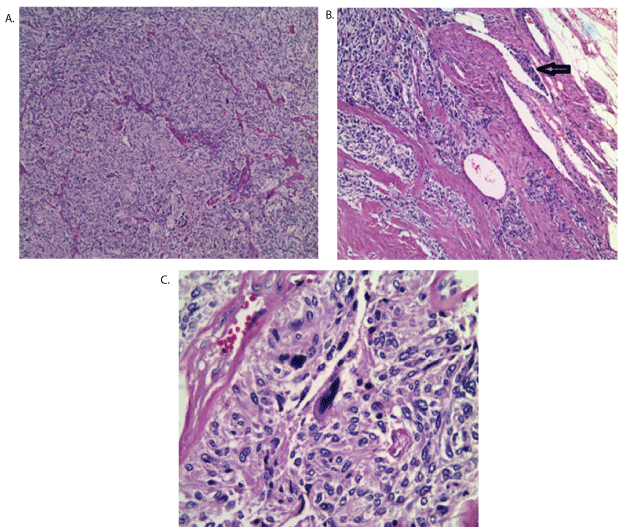
Figure 2: A-10X zoom-Hematoxylin and eosin protocol showing nuclear atypia, pleomorphism and hyperchromatic nucleus. B-Vascular invasion and multiple necrosis zones. Arrow indicates malignancy aspect. C-40X zoom-Cellular atypia with marked pleomorphism.
We performed a Scintigraphy using I-131-metaiodobenzylguanidine (I-MIBG) after surgery with results of “negative for surgical limits and other possible tumors”; the genetic and familiar analysis because of multiple paragangliomas history is in progress.
Paragangliomas are tumors derived from neuroendocrine tissues; they can be found in the cervical, thoracic, and abdominal spaces, usually paravertebral, and often they are present in the adrenal medulla and are referred to as phaeochromocytomas [1]. Outside the adrenal gland, these tumors are thought to be derived from either the parasympathetic or symphatethic system [1].
Those located in the lower mediastinum, retroperitoneum and the adrenal medulla are generally secretor and functional tumors [1]. Distal locations surrounding arterial structures such as aortic bifurcation or iliac arteries are rare, as is the involvement of other pelvic organs such as the urinary bladder [1,2].
Their most frequent location is at the Zuckerland organ or “abdominal paraganglium”, which is located beside the origin of the inferior mesenteric artery; it is a secondary source of catecholamine in the adult and functions similar to the suprarenal glands [1].
Cervical paraganglioma, generally non-malignant, can have and invasive behavior with local metastasis up to 5%; there are present as multiple forms in 4% and can be familiar even in 10% of the cases. There are no established criteria to determine its malignant character; however, capsule invasion, tumor bigger than 5 cm, weight more than 80 g, recurrent disease or metastasis can indicate so. In contrast to phaeochromocytomas, which have a metastatic disease prevalence of 5%, paragangliomas can have up to 33% metastatic disease at diagnosis [3].
Other factors to determine recurrence or increased malignant risk include tumor location, number of tumors, size, histopathological characteristics, familiar history and patient age (younger patients usually have larger tumors). Recurrence is more common, up to 33%, in nonadrenal tumors rather than in suprarenal tumor, 14%, and in those cases of familiar disease it can be up to 33% against the 13% expected in sporadic cases [3].
Non-adrenal paraganglioma at retroperitoneum are usually diagnosed between age 30 and 50 and do not have gender predisposal. They are unique in 85% of cases, frequently below-kidney, functional in 25 to 60% and cause blood hypertension as a main symptom, malignancy incidence is between 20 and 42% [4].
Urinary bladder paraganglioma represents less than 0.05% of tumors that are urothelium non-dependent and 10% of those non-adrenal paragangliomas. Up to 15% are malignant, mostly in females. Since 1953, less than 200 cases have been reported as paragangliomas [5].
In contrast to parasympathetic paraganglioma, the sympathetic paraganglioma are functional in 86% and only 10% are located at urinary bladder.
They are vascularized tumors and due to its functional character, procedures as diagnostic biopsies hold a high risk of bleeding or adrenergic shock. Those involving familiar syndromes can be associated with other tumors in 50% of cases and there is no specific criteria of malignancy described [6].
Clinical manifestation usually comes as a triad: macroscopically hematuria, silent in 60% of cases, paroxystic hypertension and “urinary attacks” described as having headache, tachycardia, blurry vision and profuse sweating during or after urination due to urinary bladder distension, sexual activity, ejaculation or instrumentation [7].
An inherited factor is mentioned in 30% of the cases; however, other series do not report any correlation [5]. They have a malignant behavior in 5 to 19% of the cases regarding paragangliomas, but it can’t be foreseen due to histological findings such as invasion, necrosis or mitosis, so with no chemotherapy or radiotherapy response, partial cystectomy its recommended [8]. Total cystectomy only for malignant tumors has been recommended [9].
Common symptoms are hematuria, blood hypertension, headaches, abdominal pain and tachycardia. When having macroscopically hematuria is important to consider a probable urothelium neoplasm [7]. During cystoscopy it can be observed as a submucosal lesion, highly vascularized, typically located deep at the urinary bladder wall, and when it can be measured usually it is affecting muscular is propia layer [9]. Diagnosis its best made when proving metanephrin presence with sensibility and specificity up to 97%, however, in atypical cases only histopathological diagnosis is definitive.
A percentage between 10 and 30% is incidentally diagnosed, by image study or by metabolite determination such as urinary normetanephrine, urinary metanephrine or serum metanephrineor dopamine increase with a 99% sensibility and 89% specificity [7,10]. It is mentioned that for low risk patients urinary test should be made, and for high-risk patients serum test should be made [7].
Biochemical testing is recommended in patients with unexplainable variability of blood pressure, paroxysmal events, diagnosis of adrenal incident aloma of predisposition for hereditary phaeochromocytoma or paraganglioma [8,11]. Although sampling conditions are very important, and discussed afterwards, measurements of urinary and plasma catecholamines are insufficiently reliable due to episodic secretion by paragangliomas or phaeochromocytoma, or even no detectable in asymptomatic patients [7,8,11].
Computerized axial tomography of the abdomen and pelvis has a 93- 100% sensibility in adrenal tumor detection and 95% in non-adrenal tumors, with a lower specificity about 60% [7]. Magnetic resonance imaging (MRI) can have similar sensibility, same low specificity 50%, but it has the advantage of no radiation, no iodated contrast and less chance of renal injury when adequate gadolinium isotopes are used [11]. T2 phase imaging gives an excellent size definition, vascular relations and metastasis location with 100% sensibility [11]. Scintigraphy using I-131- metaiodobenzylguanidine (I-MIBG) has a sensibility of 80 to 95% and specificity of 100%, useful in metastasis detection [12].
Positron emission tomography (PET) promises good results using 18F-fluorodeoxyglucose (FDG) or 6-18F fluordopamine [11] and C-hydroxiephedrine [12]. Histopathological description shows big polygonal cells, eosinophilic granular cytoplasm and rounded nucleus [7].
Radiotherapy as a palliative care for metastasis and lack of response to chemotherapy has been described; although has been reported good response with chemotherapy, it is still not well demonstrated and lacks standardization. Usually it involves a 16-month survival after metastasis diagnosis [5].
Chemotherapy using cyclophosphamide, vincristine and dacarbazine reports a partial response concerning tumor volume in about 37% of patients and a partial response con catecholamine excess in about 40% of patients [11,13,14]. Surgical resection is the preferred treatment. Preoperative planning is vital to avoid either hypertensive crisis or vascular collapse due to sudden catecholamine production cease, as most of these tumors are functional [11]. It should involve administration of alpha-blockers 7 to 10 days before surgery and betablockers afterwards.
There is no standardized type of surgery as this is a low-frequency disease. Partial or radical cystectomy, even by open or laparoscopic approach is suggested, and its realization depends on tumor size, location and surrounding tissue involvement [7]. Surgical resection is recommended as treatment; considering size, tumor volume, bladder involvement, patient age, co-morbidities and quality of life it can be performed as partial or radical cystectomy, both with life-long follow-up due to high recurrences [6,7,12].
Transurethral resection of the bladder tumor for small paragangliomas or for those in the intra-mural portion of the bladder wall has been described, although it may result in fluctuation of the blood pressure [6,7].
Discussion
Paragangliomas are tumors derived from neuroendocrine tissues. Depending on its size and anatomical location they can cause a variety of signs and symptoms but its morphology it is generally the same [15].
Non-adrenal paragangliomas are tumors with chromaffin cells, with origin at the sympathetic and parasympathetic non-adrenal systems and can be classified in 13 types according the World Health Organization (WHO), being extremely rare at iliac arteries, cauda equine, urinary bladder or duodenum [16].
Paragangliomas of the iliac artery are extremely rare, to our knowledge they are only 2 case reports of this tumors; those involving the urinary bladder represent less than 0.06 of urinary bladder tumors and less than 1% of phaeochromocytomas [17].
Almost 80% of the urinary tract paragangliomas are located at the urinary bladder and the least common are involving ureters [16], they can be functional and can cause hematuria, blurry vision, headaches or syncope during urination, or they can be nonfunctional [13].
At MRI, they are characterized by high signals and it is the preferred study of choice due to its capacity to detect small paraganglioma or those of difficult location; however, diverse drugs that affect catecholamine levels (anti-depressives, anti-flu medication, Paracetamol, Reserpine, Levodope, Ethanol or cocaine) can interfere with imaging studies [8,11].
MIBG is a guanethidine analogue which structure is very similar to norepinephrine and can be accumulated in lesions that have an adrenergic origin. It can also be accumulated in adrenergic tissue of the plasmatic membrane and capitation is sodium-dependent, so it can give falsepositives at MRI [14,18].
All previously mentioned drugs should be suspended 3 days before the study is made; in order to avoid suppression syndrome, 30 days is recommended [14].
Scintigraphy using I-131-metaiodobenzylguanidine (I-MIBG) is recommended due its high sensibility and in those patients with biochemical evidence, adrenal tumor bigger than 10 cm or metastatic disease suspicion, adding lesions that are no visible using CT or MRI [7].
Surgery performed in lesions involving arterial structures should be resectioned with previous medication if they are functional, in order to avoid catecholamine rush and adequate circulatory volume to avoid shock. Urinary bladder lesions do not have a “type of procedure” consensus; it can be either partial or radical cystectomy by open or laparoscopic approach, sometimes involving lymphadenectomy. All these depend on size, location and surrounding tissue involvement; histopathological trans-surgical studies are recommended to avoid recurrent disease [7].
Korpershoek et al. (Cancer, 2007) recommend genetic study in patients with non-adrenal, bilateral or multiple tumors. These lesions have been associated with syndromes such as multiple endocrine neoplasm, neurofibromatosis, Von Hippel-Lindau disease or Carney triad. It is mostly certain that when multiple tumors are found, they are malignant [18].
In our case, we could not establish if they were two separate tumors or a urinary bladder tumor with metastasis, due to the lack of communication between hospitals and care-physicians; we only had slides involving a urinary bladder lesion and concluded that it was either paragangliomatosis or iliac artery metastasis from urinary bladder paraganglioma.
Conclusions
Being such a rare disease, we suggest approaching each patient individually; surgical procedure is recommended with preoperative planning involving medication and imaging analysis. Catecholamine determination is vital in asymptomatic patients. Serum metanephrine tests are the most sensitive and specific. Genetic study in multiple paragangliomatosis is mandatory. MRI is the preferred study to diagnose multiple or small tumors.
Authors declare no conflict interest.
Download Provisional PDF Here
Article Type: Case Report
Citation: Bizueto-Rosas H, Pérez-González HA, Gómez-Calvo CD, Hernández-Rivera LF, AburtoPérez R, et al. (2017) Urinary Bladder and Left Hypogastric Artery Paraganglioma. A Case Report and Literature Review. J Surg Open Access 3(3): doi http://dx.doi.org/10.16966/2470-0991.154
Copyright: © 2017 Bizueto-Rosas H, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Publication history:
All Sci Forschen Journals are Open Access