Introduction
Gambling has become a widespread and very popular recreational
activity, often involving very significant sums of money being gambled
across a wide variety of populations [1]. While for most, gambling
represents an enjoyable social activity with no associated problems, for
perhaps as many as 5% of the population, gambling-related problems
can develop [2]. When these problems reach clinical significance they
are termed “Gambling disorder” [3]. Generally, research has been carried
out in those who have the clinical condition of gambling disorder, and
usually with individuals whose gambling occurs in casinos, bingo halls,
horse racing, sports betting, or using electronic video-lottery machines
[1]. However, one example of gambling behavior has frequently been
overlooked in research, in part as it isn’t always considered as gambling,
namely investing by individuals in the stock market. Nonetheless, it has
been repeatedly suggested that investing in the stock market has many
obvious parallels to gambling [4,5]: both involve making decisions under
conditions of uncertainty and both can have major financial consequences.
While there are many studies of gambling behavior, understanding
gambling via research utilizing a stock market investment perspective is
relatively unusual. Furthermore, although most research has been carried
out on those who meet clinical criteria for a gambling disorder, subclinical
gamblers are relatively poorly studied.
Neurobiological studies of gambling behavior have reported findings
similar to those from other addictions such as substance abuse [6].
A common theme has emerged in much of the literature: diminished
activation in the ventromedial prefrontal cortex (vmPFC)/orbitofrontal
cortex (OFC) during gambling and exposure to gambling cues [2]. Other
areas of interest include the ventral striatum [7,8], insula [8-10], dorsal
anterior cingulated cortex (ACC) [11], ventrolateral prefrontal cortex
(vlPFC) [12], and thalamus [11]. In the current study, we wished to
examine if any of these differences also appear with subclinical gamblers,
or if they have their own unique neurobiological effects.
Taking both economics and theories of the neurobiology of addictions
and gambling into account, the present study compares sub clinical
gamblers to non-gamblers in an investment task during functional
magnetic resonance imaging scanning (fMRI).
In the current study, we wished to examine if the differences consistently
found in pathological gamblers also appear with subclinical gamblers, or
if there are unique neurobiological effects. Based on the previous literature
we had several hypotheses:
- We predicted that subclinical “Gamblers” would differ from
“Controls” on the overall task and overall feedback phases of the
task. We anticipated regional differences in the vmPFC and the
striatum (reward pathway regions).
- We hypothesized that “Gamblers” would perform poorly on the task
compared to “Controls”, as indicated by lower financial outcome in
the task. This would be similar to previous studies which have found
dysfunctional decision-making patterns made by pathological
gamblers. Additionally, we expected “Gamblers” not to be as
obedient/affected by the advice presented during the task compared
to “Controls”, and to follow “expert” advice less frequently
- Since we do not expect “Gamblers” to follow the advice provided by
the “expert”, we hypothesized that there would be no differences in
brain activation when comparing Advice trials to No Advice trials
for the group of “Gamblers”, but that differences would emerge
when comparing “Gamblers” to “Controls”. Regions of hypothesized
differences were the ACC and prefrontal cortex.
Materials and Methods
Ethics statement
This study was approved by the Ethics review board of the University
of Alberta. All participants signed an informed consent form. As a
standard for ethical research the Ethics review board requires that
individuals are not paid substantially more than the minimum wage, to
prevent inappropriate incentives for research. For this reason, the possible
financial incentives available to individuals were at least $45 (representing
$15 per hour for their time, including screening and actually taking part in
the task). Nonetheless, it was accepted as ethically appropriate to have an
additional monetary award available that would make participation more
realistic, and after discussion with the Ethics committee the maximum
additional amount any individual could theoretically obtain was limited
to an additional $60, although the expectation was that it would be much
less in almost all cases.
Recruitment and study process
Participants were recruited from the University of Alberta Campus and
surrounding area via online advertising. A total of 39 individuals entered
the study (mean age 26.13 ± 6.23 years, range: 28 years) of which 74.4%
were male. Based on the scores on the Problem Gambling Severity Index
(PGSI), there were 23 individuals in the Control group and 16 individuals
in the “Gambler” group.
Prior to participation all participants were screened for any potential
metal in their body, since this is an MRI exclusion factor. Other
exclusion criteria included a history of Axis I psychiatric disorders,
following interview to determine a history of any disorder meeting
DSM-IV-TR criteria [13], including substance abuse or any Axis 1 or
Axis 2 mental health disorder. After screening, participants were asked
to attend the NMR Centre for a 2-hour session in which the scanning
was completed. Participants were compensated for their time (min $45)
with the opportunity to earn additional money ($45-$105) depending
on task performance (the more money earned in the task the more the
participants would receive).
Gamblers
Participants gambling behavior was assessed using the Problem
Gambling Severity Index (PGSI) [14]. Participants were asked to think
about the last 12 months and answer nine questions (e.g. Have you bet
more than you could really afford to lose?), scoring from 0 (never) to 3
(almost always). Responses for all questions were summed for a total scale
score of problem gambling. Total scores of 3-7 indicate a moderate level
of problems due to gambling, leading to some negative consequences.
It should be noted that a recent study on the validity of the PGSI found
that the differences between low and moderate-risk categories on the
PGSI was not statistically significant across all tested dimensions and that
a suggestion to combine the two groups to improve discrimination did
emerge [15]. However, the study did conclude that this group did differ
significantly from problem gamblers and thus we are confident that our
cut-off scores were appropriate.
Investment task
Prior to participation in the fMRI scan, participants were trained on
the study investment task (Figure 1), which we have utilized in previous
research [16]. Participants were informed that the investigators were
interested in investment decision-making. Participants were shown a
series of stocks, each presented individually. These showed the probability
that the stock would win a specified amount of money, but also provided
the risk that each stock could also lose a specified amount of money.
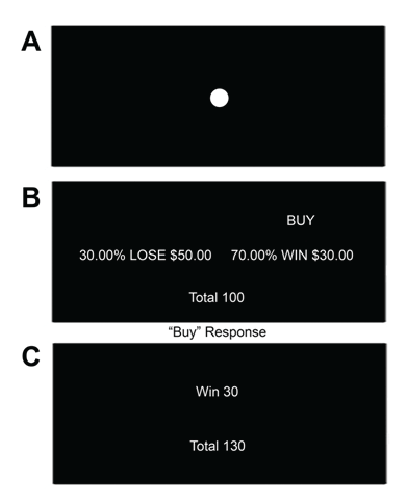
Figure 1: Investment task-a: Fixation Point (6-10s): Participants were
instructed to attend to the fixation point b: Trial (7s): Participants are
presented with a stock and must decide to either “Buy” or “Not Buy”.
Advice to “Buy” is rational as the expected value of buying the stock (0.7 ×
30=21) outweighs the expected value of not buying the stock (0.3 × -50=-
15). c: Feedback (1s): Participants are presented with feedback based
on their decision (in this case the participant chose to obey the advice and
“Buy” thus the trial resulted in a win) and their total is adjusted accordingly.
At the initiation of the fMRI study, all participants began with a
nominal amount of $100. This was chosen to be realistic in context of what
they were receiving for their time, and that any changes would be relevant
and realistic compared to this sum ($45). They were instructed to indicate,
via one buttons in each of their hands, whether they would like to “Buy” or
“Not Buy” the stock. If a stock were bought, the participant would receive
immediate feedback on whether or not that decision yielded a win, or a
loss, and their total would be adjusted accordingly. If the stock were not
bought, the participant would not receive feedback, and their total would
remain unchanged. In order to earn a higher payout post task (ranging
from $45-$105), participants were required to earn as much money as
possible throughout the task. Each participant was allowed a practice run
of the task that was equivalent in length to the first run that they would
complete in the MRI scanner. During the practice run of 19 trials,
seven were “No Advice” trials while the remaining 12 were all “Good
Advice” trials.
Participants were told that in order to simulate real-world investing,
advice was going to be presented with some of the trials. All participants
were told that the advice came from an outside financial expert, with
over 20 years of experience in the financial field, who had been asked to
indicate what advice he would give to his clients for each stock in the task.
In reality, the advice was manipulated throughout the entire task, as was
the outcome of each trial, with each result based on its’ expected value.
Thus, the “advice” was helpful, or “correct” if there was a greater chance of
the expected value being positive. For example, if the information shown
was a 30% chance of losing $50 (which would be $15) was less than the
expected value of 70% chance of winning $30 (which could be $21) then
the “correct” advice would be to “buy” (Figure 1). If the subject followed
the advice then they would have made the appropriate decision and the
amount they had would increase by the total amount available (in this
case $30). However, the advice was gradually changed during the course
of the task, starting initially where all advice was correct but by the end
all the advice was incorrect. Thus, during the first trials the advice would
be correct in all cases, and would correctly prompt participants to “Buy”
stocks that would yield wins and increase their money. Similarly, during
all of the early trials any advice to a participant to “Not Buy” stocks would
prevent them losing money. Thus, if the participant followed the advice on
all occasions during the first part of the study task then their money would
increase, and they would not lose any. However, and unknown to the
participants, after 1/3 of the tasks had been completed the advice changed
to having an equal mix of either corrector being given incorrect advice.
Therefore, should individuals follow the advice in the first 1/3 of tasks they
would make money, and during the next 1/3 of tasks they would neither
win nor lose overall. However, in the final 1/3 of the tasks, again with no
indication of a change to the participants, the advice changed so that it was
always incorrect. Thus, if individuals followed the advice during the final
1/3 of the task they would always lose money (Table 1).
| Trials |
Duration of Run |
Type of Advice |
Type of Buy |
Number of Trials |
First 1/3 of trials
Runs 1 and 2 |
5 min 30 sec |
No Advice |
Good Buy |
8 |
| Bad Buy |
6 |
| Good Advice |
Good Buy |
12 |
| Bad Buy |
12 |
Second 1/3of trials
Runs 3 and 4 |
9 min |
Good Advice |
Good Buy |
18 |
| Bad Buy |
16 |
| Bad Advice |
Good Buy |
16 |
| Bad Buy |
16 |
Last 1/3of trials
Runs 5 and 6 |
5 min 30 sec |
No Advice |
Good Buy |
8 |
| Bad Buy |
6 |
| Bad Advice |
Good Buy |
12 |
| Bad Buy |
12 |
Table 1: Investment task conditions
Image acquisition
Scanning took place at the University of Alberta’s Peter S. Allen MR
Research Centre using the 1.5T Siemens MRI system with an 8-channel
head coil. Thirty-two axial slices (3 × 3 × 4 mm voxels) were acquired
in a descending interleaves order. Functional images were acquired using
a gradient echo EPI sequence (TR=2000 ms, TE=40 ms, FOV=256 mm,
flip angle=90°). Structural images were acquired with a T1-weighted
pulse sequence (MPRAGE, TR=1670 ms, TE=3.82 ms, TI=1100 ms,
flip angle-15°, FOV=256, 1 mm thick). Images were pre-processed and
analyzed using SPM8. Pre-processing steps included 6-parameter rigid
body motion correction, slice-timing correction, and co-registration to
each participant’s anatomical image to their functional scans. Structural
scans were normalized to the Montreal Neurological Institute (MNI)
template, and functional images were normalized to the new anatomical
image. Lastly, we performed smoothing using a three-dimensional
Gaussian filter (8-mm FWHM). Five participants (four from the “Control”
group; one from the “Gambler” group) were excluded from further
analyses due to significant movement artifacts that occurred during the
scans (pitch, roll or yaw translation greater than 8mm).
Statistical analysis
Behavioral data on the investment task was analyzed using SPSS 21.
An ANOVA and independent samples two-tailed t-test were performed
to determine differences between the groups in terms of age and gender.
To test differences in obedience between the groups Hotellings T2
test was performed on the three dependent variables: percent obedience in
Runs 1/2 (first 1/3 of trials), Runs 3/4 (second 1/3 of trials), and Runs 5/6
(final 1/3 of trials). All the study runs were grouped based on type of advice
presented), with Group (“Gambler” or “Control”) as the independent variable.
fMRI data were analyzed using the General Linear Model. Trials were
classified by type of advice (No Advice, Good Advice, Bad Advice), type
of buy (Good Buy resulting in a win, Bad Buy resulting in a loss), decision
(Buy, Did Not Buy), and feedback (Win, Lose) during model specification.
Nuisance predictors included run offsets and six motion parameters. We
included the trials from all runs in a single GLM, grouping together run
1 with run 2, run 3 with run 4, and run 5 with run 6, as per the type of
advice (Good, Bad or both) provided. GLM parameters were estimated
using linear least-squares error fitting. We computed the following firstlevel
statistical contrasts separately for each participant: Buy-Did Not
Buy, Did Not Buy-Buy, Advice-No Advice, No Advice-Advice, ObedientNot
Obedient, Not Obedient-Obedient. (Obedient and Not Obedient
trials, respectively, were defined as those in which the participant’s
choice matched/did not match the advice), Win-Lose and Lose-Win. We
performed three second level analyses on the amplitudes of each contrast:
within group t-test across all participants in the “Control” group to detect
significant contrast amplitude, within-group t-test across all participants
in the “Gamblers” group, and between-groups t-test comparison. For
all analysis, we used a voxelwise statistical threshold of t(37)=2.0262
(p<sss0.05 uncorrected) and a cluster size threshold of k=201 voxels,
yielding p<0.05 corrected for multiple comparisons across both the voxel
population as well as the statistical tests. Cluster size threshold level was
computed using Monte Carlo simulation. Post-hoc analysis of reactiontime
data was conducted using SPSS 21.
Results
Behavioral results
There were no statistically significant differences in age or gender
between the two groups; however, as required by the design, gamblers
scored significantly higher on the PGSI (t(37)= -8.160, p<0.0001).
The assumption of equality of covariance matrices was satisfied for our
two-group MANOVA (Box’s M=6.44, p=0.44). There was a statistically
significant difference between the groups (Gamblers and Non Gamblers)
on the combined dependent variable (Run), (Hotellings T2
=11.25,
F(3/35)=3.549, p=0.024; Note. T2
=Trace coefficient × (sample size-number
of groups)=0.304 × (39-2)=11.25). Post-hoc univariate ANOVAs were
conducted to determine the effect of group on each of the Runs (Figure
2). A significant difference between the groups only appeared in Runs 5/6
(F(1,37)=5.416, p=0.026). Both runs 1/2 (F(1,37)=0.63, p=0.43) and 3/4
(F(1,37)=0.144, p=0.71) failed to reach statistical significance.
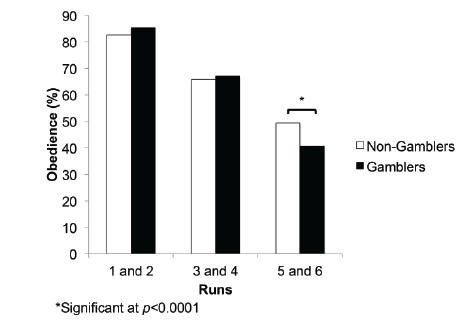
Figure 2: Comparison between Non-Gamblers and Gamblers in
obedient decisions-Significant differences in number of obedient
decisions in the final two runs of the study (F(1,40)=16.254, p<0.0001).
The “Expert” group was significantly more obedient to the advice in the
last two runs than the “Peer” group.
Task performance: Total monetary score at the end of the task
determined task performance with higher performance indicated by
a higher total score. There were no statistically significant differences
between the groups in terms of task performance (t(37)= -1.872, p=0.069,
Cohen’s d= -0.625).
Reaction times: Reaction time analyses revealed no statistically
significant differences in overall reaction times throughout the task
between groups.
Significant reaction time ANOVAs:
2(Group; Non-Gambler, Gambler) × 2(Obedience; Obedient, Not
Obedient) ANOVA
A main effect for Obedience emerged (F(1,72)=8.124, p=0.006) with
Not Obedient (M=3.574 seconds, SD=0.757 seconds) decisions taking
longer than Obedient decisions (M=3.135 seconds, SD=0.596 seconds).
There was no main effect of group or interaction effect.
2(Group; Non-Gambler, Gambler) × 2(Good Advice Obedience;
Good Advice Obedient, Good Advice Not Obedient) ANOVA
A main effect emerged for Good Advice Obedience (F(1,72)=14.776,
p<0.0001) with Not Obedient (M=3.717, SD=0.828) decisions being
slower than Obedient (M=3.073, SD=0.605) decisions when the advice
presented was good.
2(Group; Non-Gambler, Gambler) × 2(Advice; Good Advice, Bad
Advice) ANOVA
A main effect approached significance for group (F(1,72)=3.178,
p=0.079) with Gamblers (M=3.509 seconds, SD=0.117 seconds) being
slower than Non-Gamblers (M=3.236 seconds, SD=0.099 seconds). There
was no main effect of Advice or interaction effect.
Neuroimaging results
Overall task: There were no group differences when comparing
overall task activation during all presentation/decision phases of the task.
Differences in activation did emerge when comparing the two groups
during the feedback phase of the task. Thus, “Gamblers” displayed greater
activation in bilateral insula, thalamus and dorsal-medial prefrontal
cortex compared to non-gamblers (Figure 3).
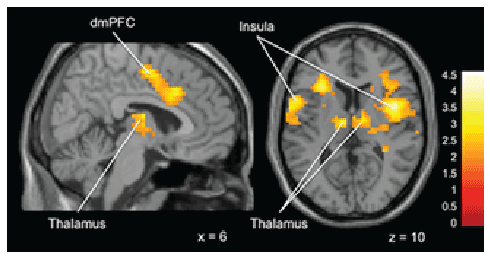
Figure 3: Brain activation for statistical contrast maps Overall Task
Feedback Phase -“Gamblers” show increased activation in bilateral
insula, thalamus and dorsal-medial prefrontal cortex. “x” and “z”
coordinate provided at bottom right corners in MNI space. All results
voxelwise statistical threshold at t=2.0211 (p<0.05) and a cluster
threshold level of k=201, p<0.05 corrected for multiple comparisons.
Obedient vs Not Obedient: Statistically significant differences emerged
when comparing Obedient to Not Obedient trials in the middle runs,
3 and 4 (when subjects received a mix of good advice and bad advice).
During these study runs there was significant activation compared to
baseline for Obedient compared to Not Obedient trials in “Gamblers”
in the left inferior parietal lobule, insula, medial frontal gyrus and the
ventral anterior cingulate cortex compared to “Non-Gamblers” (Figure 4).
There was significant activation compared to baseline for Not Obedient
compared to obedient trials in “Non-gamblers” in both the dorsal and
ventral anterior cingulate cortex compared to “Gamblers”. In contrast,
no statistically significant differences between the groups emerged when
comparing the first two runs (1/2) (all advice correct) and the final two
runs (5/6) (all advice incorrect).
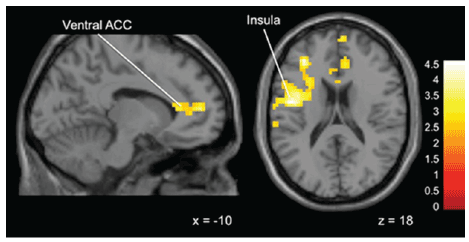
Figure 4: Brain activation for statistical contrast maps OBEDIENT–NOT
OBEDIENT in Sub-threshold Gamblers-“Gamblers” display greater
activation in the ventral ACC and insula. “x” and “z” coordinate provided
at bottom right corners in MNI space. All results voxelwise statistical
threshold at t=2.0211 (p<0.05) and a cluster threshold level of k=201,
p<0.05 corrected for multiple comparisons.
Advice vs No Advice: Differences emerged between “Gamblers”
and “Non-gamblers” when comparing Advice to No Advice trials, with
“Gamblers” displaying significant activation in the superior frontal gyrus
and the anterior cingulate gyrus compared to baseline during Advice trials
(Figure 5). At the within-groups level, differences between Advice and No
Advice trials did emerge in “Gamblers” with Advice trials recruiting the
occipital lobe and No Advice trials recruiting the left putamen and left
precentral gyrus (Figure 6).
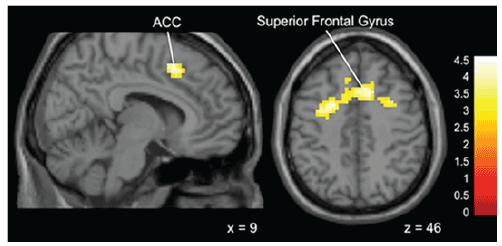
Figure 5: Brain activation for statistical contrast map ADVICE–NO
ADVICE in Gamblers-“Gamblers” show greater activation in the superior
frontal gyrus and anterior cingulate gyrus compared to non-Gamblers.
“x” and “z” coordinate provided at bottom right corners in MNI space. All
results voxelwise statistical threshold at t=2.0211 (p<0.05) and a cluster
threshold level of k=201, p<0.05 corrected for multiple comparisons.
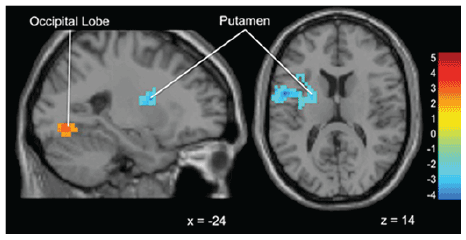
Figure 6: Brain activation for statistical contrast map ADVICE–No
ADVICE (Within groups sub-threshold gamblers)-At the within groups
level, “Gamblers” show activation in the occipital lobe during Advice
trials and greater activation in the putamen in No Advice trials. “x”
and “z” coordinate provided at bottom right corners in MNI space. All
results voxelwise statistical threshold at t=2.0211 (p<0.05) and a cluster
threshold level of k=201, p<0.05 corrected for multiple comparisons.
Good Advice vs Bad Advice: No significant differences emerged
between groups when comparing Good Advice trials to Bad Advice trials.
No significant difference emerged between Good and Bad Advice trials
when combining the groups together.
Buy vs Did Not Buy: Differences emerged when comparing Buy to
Did Not Buy trials. “Gamblers” displayed significantly greater activation
in the bilateral precuneus while “Non-gamblers” displayed significant
deactivation in the posterior cingulate cortex and right inferior parietal
lobule in Buy compared to Did Not Buy trials. “Non-gamblers” showed
significantly greater activation compared to baseline in the posterior
cingulate gyrus in Did Not Buy compared to Buy trials.
Win vs Lose: Significant differences emerged when comparing
Win feedback and Lose feedback. “Non-gamblers” showed significant
activation while “Gamblers” showed significant deactivation compared to
baseline in the right inferior frontal gyrus, bilateral medial frontal gyrus
and right insula when receiving Win feedback. There was significant
deactivation compared to baseline in the right thalamus and right
superior frontal gyrus in “Gamblers” while “Non-gamblers” displayed
significant activation in the left insula and right dorsal medial prefrontal
cortex compared to baseline when receiving Win feedback.
Discussion
Overall decision-making
In the present study we were interested in examining how subthreshold
gamblers respond during our investment task and how this
manifests itself neurobiologically. Based on previous research it has been
established that pathological gamblers frequently show deactivation in
the vmPFC during gambling tasks. Interestingly, however, this pattern of
activation was not found in the present study in the group who had subthreshold
gambling, and also there no statistically significant differences
in the overall decision-making phases of the task between controls and
those with sub-threshold gambling.
In a previous fMRI study, a decrease in the ventral striatal and
ventromedial prefrontal cortex (vmPFC) activation during receipt of
monetary rewards was found in pathological gamblers compared to
healthy controls [7]. Additionally, the authors found that there was a
negative correlation between the severity of gambling problems and
activation in the ventral striatum [7]. These results support the hypothesis
that gamblers may have a decreased reward sensitivity compared to
non-gamblers. Similarly, one group found that pathological gamblers
performed poorly compared to nicotine dependent men and healthy
controls during an affective switching task [12]. In this other study,
participants were asked to respond to one of two stimuli presented at each
trial and were then given either positive or negative feedback (8:2 ratio)
and pathological gamblers performed the worst, followed by nicotine
dependent men, with healthy controls performing the best [12]. Given
these findings, it would have been anticipated that the sub-threshold
gambling group in the present study would have been similar to previous
findings from studies in problem gamblers, but in fact this is not what
we found.
Similarly, cue reactivity has been associated with cravings and
attentional bias to addiction-related stimuli, and is also a central
characteristic of pathological gambling [17-19]. Potenza et al. [20] were
the first to conduct an fMRI study on gambling urges. They tasked
their participants with viewing a tape designed to evoke emotional and
motivational cues to gambling. The pathological gambling group exhibited
less activation, compared to healthy controls, in the cingulate gyrus, OFC,
caudate, basal ganglia and thalamic areas [20]. Another group, utilizing
a similar gambling movie paradigm, found an increased BOLD signal
in the right dlPFC, right inferior frontal gyrus, medial frontal gyrus,
left parahippocampal region and left occipital cortex when pathological
gamblers are presented with gambling-related cues [21]. Again, given
these findings, it would have been anticipated that in the present study the
group of sub-threshold gamblers would have had similar brain changes
during this paradigm, but it is not what we observed.
It can be seen that previous research with pathological gamblers
suggests that gamblers display dysfunctional brain activation in several
regions during decision-making including the ACC, OFC, vlPFC, NAcc
and amygdala [22-25]; however, these patterns were not replicated with
sub-threshold gamblers. Thus from our findings it is possible that these
areas, typically dysfunctionally recruited in pathological gamblers, may
represent regions associated with the severity of gambling behavior.
In contrast, when the task was analyzed to compare “Buy” with “Did
Not Buy” decisions, statistically significant differences did emerge. Thus,
Sub-threshold gamblers displayed activation in the precuneus during
“Buy” trials compared to non-gamblers. The precuneus has been linked
to episodic memory [26], and it is therefore possible that in this group of
sub-threshold gamblers decisions to “Buy” may conceivably be triggered
by context-related (gambling) memories. Furthermore, deactivation in
the posterior cingulate cortex may signify a lack of emotional salience
associated with choosing to gamble/buy a stock in non-gamblers, since
as this region has been linked to memory of emotional stimuli [27]. A
suppression of activation in this region suggests that the decision to buy
a stock in this task does not present strong positive or negative emotional
valence in non-gamblers compared to sub-threshold gamblers. One,
potentially speculative explanation, would be that decisions to gamble are
not as strongly encoded in memory for non-gamblers. Another possibility
could be that, in this stock market investment task, bounded rationality
may have impacted an individual’s decisions. Bounded rationality refers
to the use of cognitive shortcuts in decision-making when uncertainty
is present, and where the cost of gathering information and solving a
problem exactly are far greater than the value of such an exact solution
[28,29]. It can therefore be seen that studying sub-threshold gamblers, and
not just those with gambling disorder, may open potential new approaches
to consider regarding the mechanisms for underlying changes that occur
in those with a gambling disorder.
Our overall task analysis yielded differences between the two groups
(gamblers and non-gamblers) during the feedback phase, partially
supporting our first hypothesis, indicating that some of the reward
pathways (insula and thalamus) may be affected in those who gamble but
do not meet the criteria for Gambling Disorder, at least when compared
to those who do not gamble. This suggests that it is conceivable that subthreshold
gamblers may have dysfunctional reward pathways compared to
non-gamblers, at least in regards to financial decision-making. This could
potentially comprise part of the mechanism that leads to continued and
increased levels of gambling. Interestingly, when comparing Win feedback
to Lose feedback, several differences emerged between the groups. The
inferior frontal gyrus has been implicated in GO/NO-GO tasks, and is
believed to be involved in response inhibition [30-32]. Thus, a dampening
of activation in this area would be consistent with the possibility that after
sub-threshold gamblers are presented with winning feedback, they may
be less likely to ‘stop while they are ahead’, whereas a win may signal when
it is time to quit in non-gamblers. Such a suggestion is also compatible
with some findings from previous studies. For example diminished
activation in gamblers compared to controls in response to reward has
been suggested [7,8], and our results support this finding with regard
to the insula. Our findings also suggest that one of the key elements
separating sub-threshold gamblers and pathological gamblers may
be the addition of decreased activity in both the vmPFC and ventral
striatal area [7,8]. This in turn could lead to greater reward processing
dysfunction, followed by increased likelihood of development of
Gambling Disorder.
Performance and obedience
Our second hypothesis was that sub-threshold gamblers would perform
poorly compared to controls, but this was not supported by our findings.
This would imply that in sub-threshold gamblers decision-making is not
significantly impaired. In marked contrast, previous work has suggested
that pathological gamblers perform significantly worse, often by taking
higher risk options, on a variety of tasks including the Iowa Gambling Task
(IGT) [33-36], Game of Dice task (GDT) [37,38], and the Wisconsin Card
Sorting Test (WCST). Additionally, one study determined that the nucleus
accumbens (NAcc) tracks price bubbles in stock markets as well as level
of aggressiveness in trading [5]. The authors found that this aggressive
(based on NAcc signals) trading earned less overall, with more successful
traders having an “early warning system” signal from the anterior insular
cortex when stock prices reach a peak, leading these traders to sell their
stocks prior to a crash. It is suggested that examining the signals from the
NAcc might demonstrate an individual’s “irrational exuberance” and that
such methods could be applied to gamblers as well [5].
Our results suggest that this harmful decision-making pattern is not
significantly present in our gambling group (who were sub-threshold).
This finding is in keeping with the lack of significant findings in
brain activation in the overall task, as with this lack of significant
neurobiological difference we may not see any significant behavioral
differences.
However, part of our second hypothesis was supported, namely that
we expected “Gamblers” not to be as obedient/affected by the advice
presented during the task compared to “Controls”. Thus, we found that
obedience to the presented advice did differ between the groups, with
“Non-gamblers” making more obedient decisions than “Gamblers” on
the overall task. When examining the task during each successive run,
we found that the groups differed significantly in the final 1/3 of the task,
when the advice being given was always incorrect. It is of great interest that
our results imply that the gamblers were, in fact, making more rational
decisions than the controls, and that their decision-making was superior
to that of the non-gambling control group. Nonetheless, this more
rational decision-making during the final 1/3 of the task was not enough
to significantly improve final performance/financial outcome, as neither
group outperformed the other. These findings provides some evidence
that not all gambling behavior leads to irrational decision-making, and
that it is conceivable that a moderate degree of gambling propensity
could potentially shield investors from following poor advice, and in the
long run result in more profitable decisions. However, such a suggestion
is speculative at present, even though differences in obedient decisions
between the two groups continued to grow larger throughout the task.
Another reason for caution was that statistically significant differences in
BOLD signal between the two groups were only found during the middle
1/3 of the task, which is at variance with the behavioral results. However
one way to explain both these findings is that it is conceivable that in the
middle 1/3 of trials, when the advice was equally mixed between correct
and incorrect advice, the sub-threshold gambling group was learning not
to trust the advice, so that for the final 1/3 of the task they were no longer
concerned with the poor advice at all, negating any large obedience or
disobedience effects. Consistent with this suggestion was our finding
that the sub-threshold gambling group recruited the insula and ventral
ACC during obedient trials, while the non-gamblers recruited the dorsal
ACC when making non-obedient decisions. In choosing to not follow the
advice of the “expert” our non-gamblers displayed activation in an area
linked to error detection [39,40] as well as violation of expectancy [41].
Meanwhile, in sub-threshold gamblers obedient trials recruited activation
in regions associated with interception [42], risk-avoidance [43,44] and
sensitivity to social and emotional evaluation [41]. The activation of the
ventral ACC may signify a desire of the sub-threshold gamblers to appear
likeable when following advice that, based on the insula recruitment, may
no longer seem sound.
Our third a priori hypothesis was that there would be no differences in
brain activation when comparing Advice trials to No Advice trials for the
“Gamblers”, but that differences will emerge when comparing “Gamblers”
to “Controls”. We further hypothesized that regions in which we would see
differences would be the ACC and prefrontal cortex. However, contrary
to this hypothesis, differences did emerge in Gamblers when comparing
Advice to No Advice trials. No Advice trials produced activation in the
putamen, which may suggest that sub-threshold gamblers associated No
Advice trials with having a greater risk, since increased risk-taking has
been associated with greater activation in the striatum [45]. However,
when comparing our two groups, greater activation in the ACC [39,40]
and superior frontal gyrus [46], during Advice trials in the gambling
group may suggest that this group was less affected by the advice. If the
advice were influencing decision-making, we would expect that in the
presence of advice there would be a decrease of cognitive effort. That this
pattern of activation was not found in the sub-threshold gamblers might
suggest that the non-gamblers made better use of the advice to a greater
extent that the sub-threshold gamblers.
Interestingly, neither group displayed differential activation between
corrector incorrect advices. This suggests that neither group really
differentiated between the two types of advice, since if this had been the
case we would have anticipated that Bad Advice trials would have
elicited greater frontal lobe activation related to a greater effort in
decision-making.
Reaction times
Post-hoc analysis of reaction times indicated that throughout the
overall task there were no significant differences between the “Gamblers”
and “Controls”. There were also no differences in reaction times when
comparing Good and Bad Advice trials, which was consistent with the
fMRI findings.
Overall, choosing not to follow the presented advice took longer than
choosing to follow it for both groups, suggesting that both were taking
the advice into account to some degree as this took greater cognitive
processing. However, this finding would also provide support for the idea
that sub-threshold gamblers were not completely ignoring the advice.
When looking at only Good Advice trials, this pattern of disobedient
decisions taking longer than obedient decisions was repeated; yet,
interestingly, this same effect was not seen when comparing on Bad
Advice trials, suggesting that when the advice was not correct participants
were able to decide not to follow it more easily.
Consistent with our fMRI results, the reaction times of sub-threshold
gamblers were slightly longer than non-gamblers during Advice trials (i.e.
they were slower) and this difference approached statistical significance. It
may suggest that in the presence of advice non-gamblers were quicker to
make their decisions, possibly due to the use of the advice as a cognitive
shortcut in their decision-making process, whereas the sub-threshold
gamblers did not make as great a use of the advice, thus requiring more
cognitive processing time before deciding what action to take.
Comparison to other recent imaging study findings
Recent fMRI studies have been somewhat supportive of the findings
described. Thus, while previous research suggested gamblers may have
dysfunctional changes in amygdale functioning [22-25], we did not find
this with sub-threshold gamblers. However, another group found that
amygdala activity varied depending upon individual differences in appetite
regarding loss aversion [47], with which may account for differences in
our findings from those of previous studies. Another group examined
brain activity in ventral striatal reward networks during decisions that
weight the utility of possible gains against possible losses. They found
that pathological gamblers had a dysregulated U-shaped response
profile, reflecting hypersensitivity to the most appetitive and aversive
bets [48]. The finding that the network is dysfunctional, particularly
for extreme bets with large potential consequences, could be consistent
with the findings from the present study that sub-threshold gamblers
associated “No Advice” trials with having a greater risk, since increased
risk-taking has been associated with greater activation in the striatum
[45]. Similarly, another group found that ventral striatal connectivity
is positively correlated with gambling severity [49] and this group also
reported that their findings corroborated the ‘non-categorical’ nature of
reward processing in gambling, where both near-misses and full-misses
are processed differently.
Finally, in terms of possible baseline differences, one group has
suggested that there are multiple differences in functional changes in
the resting state of individuals with internet gaming disorder, and that
there were several similarities to changes seen in those with alcohol use
disorder [50]. Such suggestions may suggest that there could be restingstate
changes also in sub-threshold gamblers that may link them on a
continuum of changes with other addictions. Further research is required
to determine the accuracy of such assumptions.
Conclusion
It is important to note that while the sub-threshold gamblers did
not meet the requirements for gambling disorder, which was an a
priori requirement, they still reported high levels of frequent gambling
and thus this group should still be considered regular and experienced
gamblers. We suggest that, based on the DSM-5 criteria of significant
distress for diagnosis, this lack of distress leading to overall unimpaired
decision-making of sub-threshold gamblers may have neurobiological
underpinnings that differ from both non-gamblers but also from
pathological gamblers. While overall decision-making brain region
recruitment does not differ significantly between non-gamblers and subthreshold
gamblers, the reward pathways do. It is certainly conceivable
that the vmPFC, in particular, plays an important role in the development
of Gambling Disorder, as frequent gamblers who do not meet the criteria
fail to show the pattern of deactivation so robustly found [2] in this area.
However, to understand possible differences it is suggested that future
research contain groups of healthy controls, sub-threshold gamblers,
and those with gambling disorder in order to examine differences
between these groups. Our results suggest that the inclusion of frequent
gamblers, who are sub-threshold for gambling disorder, may help greater
understanding of some of brain changes that lead to the huge societal and
individual issues caused by pathological gambling.
Acknowledgments
The authors would like to thank Andrea Shafer and Peter Seres for their
contributions to this research project.







