Abstract
A total of 120 local fresh and imported frozen broilers breast, liver, kidney and gizzard were collected from different places in Al-Menofia
Governorate, Egypt for estimation of their contents of Oxytetracycline (OTC) and Ampicillin residues by using high performance liquid
chromatography (HPLC).
Results revealed that 55%, 70%, 50%, 70%, 10% and 15% of the examined samples of local broilers meat, liver, gizzard, kidneys, imported
broiler meat and kidneys were positive for residues of OTC with mean values of 471.4 ± 33.8, 773.9 ± 49.5, 11.6 ± 0.9, 2676.0 ± 117.5, 114.7 ±
8.3 and 546.7 ± 41.2, respectively. While 50%, 55%, 35%, 50%, 35% and 45% of the same samples were positive for residues of Ampicillin with
mean values of 329.2 ± 20.7, 853.3 ± 65.1, 164.5 ± 9.8, 531.6 ± 37.4, 177.9 ± 15.4 and 323.8 ± 26.6, respectively.
Application of various cooking methods; boiling, frying and grilling on ten samples of chicken muscles indicated that cooking had an effect in
reducing the concentration of OTC residues with mean reduction % of 84.52%, 93.62% and 96.58% after boiling, frying and grilling and 81.22%,
90.54% and 94.5% after boiling, frying and grilling for concentrations of ampicillin residues.
Presence of antibiotic residues in chicken carcasses possesses a health risk to consumers such as antibiotic resistance, teratogenicity,
carcinogenicity, hepatic and renal failure. Veterinary control of withdrawal times in poultry farms and post-mortem inspection of slaughtered
carcasses for antibiotic residues are important measures to reduce the incidence of antibiotic residues in poultry meat to improve the quality of
chicken meat and to safeguard consumers.
Introduction
In poultry, antibiotics are being excessively used for various purposes
as prophylactic, control of diseases and as growth stimulants, therefore
antibiotic usage had facilitated their efficient production and enhanced
the health and wellbeing of poultry [1,2]. Oxytetracycline (OTC) and
Ampicillin are the most common routinely used in veterinary medicine
for prevention and control of diseases in poultry [3].
Unfortunately, the use of antibiotics in food-producing animals may
leave residues in food stuffs of animal origin like meat due to the failure
to observe the withdrawal periods of each drug, extra-label dosages for
animals, contamination of animal feed with the excreta of treated animals
and/or the illegal use of unlicensed antibiotics [4]. The presence of
Oxytetracycline residues in edible animal tissues has harmful effects on
consumer’s health such as allergic reactions, spreading of drug-resistant
microorganisms, liver damage, yellowing of teeth, gastrointestinal
disturbance and possible mutagenic and/or carcinogenic effects [5]. While
the most common adverse effects caused by consuming of penicillin
residues in meat were hypersensitivity reactions, especially skin rashes and
gastrointestinal disturbances including diarrhea, nausea and sometimes
vomiting [6]. Monitoring of antibiotic residues in poultry meat is
important in controlling quality and safety of foods, consequently several
analytical techniques are available for determination of antibiotic residues
in poultry tissue, the most powerful and sensitive is the technique of high
performance liquid chromatography (HPLC) [7]. Relatively simple and
rapid typical detection for the presence of multi-residues in tissues samples
could be achieved by using HPLC technique [8].
To ensure human food safety, WHO and FAO have set standards for
maximum residue limits (MRLs) in foods. Additionally, the European
Union (EU) has set own MRLs [9]. The acceptable MRLs for OTC residues
are 200ug/kg in muscle, 600ug/kg in liver and 1200 ug/kg in kidney, while
acceptable MRLs for ampicillin residues are 50ug/kg in muscle, liver and
kidney according to the Joint FAO/WHO Expert Committee on Food
Additives [10-12]. Control of the antibiotics residues in poultry meat
could be achieved by giving the drugs to the birds after sensitivity test, by
the accurate dose and prevents slaughtering in the withdrawal time [13].
Also, good cooking and freezing were used for removal of great part of the
antibiotic residues; high heat followed by sudden cold which used during
industrial processing may also remove the residues [14].
The objective of this study is to through the light on safety of the local
and imported frozen broilers tissues and giblets through monitoring
the antibiotics residues (Oxytetracycline and Ampicillin) by using the
technique of High Performance Liquid Chromatography (HPLC), with
special references to the effect of the most common cooking procedures
(boiling, frying and grilling) on the antibiotic residues level.
Materials and Methods
Collection of samples
Total of 120 broilers breast, liver, kidney and gizzard were collected
from poultry shops of different places in Al-Menoufia Governorate, Egypt
as frozen local and imported samples for determination of their residues of
oxytetracycline and ampicillin).The samples were collected in clean sterile
polyethylene bags and transferred directly to the laboratory without delay
in an ice box.
Determination of antibiotic residues in examined samples
Determination of oxytetracycline and ampicillin residues in examined
samples was applied by using High Performance Liquid Chromatography
(HPLC) technique.
Determination of oxytetracycline residues in examined samples
All samples were finely diced with scissors after trimming of the external
fat and fascia. 2 g of each organ to be analyzed were weighed using digital
balance and then cut into very small pieces and subsequently ground into
fine powder using Sartorius mincer, then homogenized in a blender for 2
min. and then 0.1 gm of citric acid was added. One ml of nitric acid (30%),
4 ml methanol and 1 ml deionized water were added to this mixture,
respectively. The suspension with solid particles was put in a vortex for
good mixing, kept in an ultrasonic bath for 15 min and centrifuged for
10 min at 5300 rpm. After filtering through a 0.45 um nylon filter, 20 ul of
solution was injected into HPLC for analysis according to Senyuva et al. [15].
Determination of ampicillin residues
Each sample was prepared for extraction of the drug with a specific
solvent
Extraction: Accurately, 5 ± 0.01 g of each sample were put into 50 ml
capped polypropylene centrifuge tube and 15 ml of acetonitrile/water
(15:2) were added. Complete homogenization of the sample for 1 minute
and centrifugation at 4000 rpm was carried out. The supernatant was taken
for repeating the homogenization and centrifugation one additional time.
Furthermore, the combined supernatants were placed into a round bottom
flask and the acetonitrile was evaporated at 37°C. At least, approximately 6
ml of remaining supernatant should be found in the flask. The total volume
of supernatant should reach 20 ml using phosphate buffer (pH 8.5). The
supernatant was filtered through regenerated cellulose, 25 mm, 45 um
syringe filter (Agilent pin 5185-5831). Actually, 10 ml of the extract were
loaded onto the Agilent Sampli Q OPT 6 mL/150 mg cartridge [16].
Purification: The cartridge was washed with 0.1% formic acid in water
and then pH 8.5 potassium phosphate buffer. Finally, the sample was
eluted with 3 ml acetonitrile. The sample was filtered with a 13 mm, 45
ml poly tetrafluoro ethylene (PTFE) syringe filter (Agilent pin 518-5836).
The eluent was dried under nitrogen at room temperature. The residue
was re-suspended in mobile phase to1.0 ml. The sample was vortexed for
2 minutes and then transferred to a 2 ml, auto sampler vial (Agilent pin
5182-0864) [17].
Solid-phase extraction and final sample preparation: The SPE cartridge
was placed into vacuum manifold system with SPE cartridge effluent going
to a solvent trap. Accurately, 25 ml of methanol and then 25 ml of water
and then 40 ml 0.01 M calcium hydroxide were added. Flow rate is not
important for these steps. Furthermore, 3ml of the sample was applied to
the cartridge with a flow rate not more than 2 drops/s. The cartridge was not
allowed to dry at this step and the cartridge was flushed with 40 ml distilled
water and then 10 ml acetonitrile. Elution was performed successively with
40 ml of 2.5% acetic acid including 50% methanol. The collected elute was
evaporated using rotator evaporator at 45°C till complete dryness. The
dried residue was reconstituted in 3 ml of the mobile phase. The samples
were mixed and filtered through 0.2 µm filters before injection into the LC system.
Chromatographic conditions
Oxytetracycline: Include a mobile phase of methanol and formic acid
0.1% using a gradient method with a flow rate of 1.5 ml/min. at 25°C.
The separation was done on Hypersil gold C18 (10 um, 100 × 4.6 mm)
columns with mobile phase as described above. Detection was performed
with PDA detector set at 350 nm wave length. Quantification of residues in
samples was obtained and calculated from areas under curves extrapolated
automatically by the software (Chromo Quest 5).
Ampicillin: High performance liquid chromatography (HPLC) used for
antibiotic determination was an Agilent 1100 HPLC system at Animal
Health Research Institute in Dokki, Agilen Technologies, Waldbronn,
Germany, equipped with quaternary pump model G 1311A, UV detector
(Model G 1314A) set at 254 nm wavelength, auto sampler (model G1329A
VP-ODS) and Shim pack (150 × 4.6 mm) column (Shimadzu, Kyoto,
Japan).
Results and Discussion
From the results reported in table (Table 1) and figures (Figure 1 and
2) it is obvious that the oxytetracycline residues were detected in 55%,
70%, 50%, 70%, 10% and 15% of the examined samples of local broilers
meat, liver, gizzard, kidneys, imported broiler meat and kidneys with mean
values of 471.4 ± 33.8, 773.9 ± 49.5, 11.6 ± 0.9, 2676.0 ± 117.5, 114.7 ± 8.3
and 546.7 ± 41.2, respectively. The current results come in accordance with
those reported by Salehzadeh et al. [18],who detected mean concentrations
of oxytetracycline (OTC) residues in chicken liver and kidney of 576.657
± 201.908 and 517.56 ± 186.64 ug/kg, respectively. The obtained results are
being higher than those obtained by Cetinkaya et al. [19] who determined
levels of oxytetracycline residues in chicken tissues of 17.2 ug/kg. The
current results are being lower than those recorded by Salama et al. [20]
who reported a maximum concentration of oxytetracycline residues in
fresh chicken breast, thigh and liver samples of 5812, 6010 and 8148 μg/
kg, respectively.
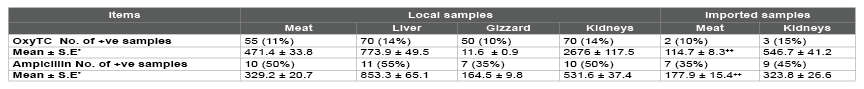
Table 1: Statistical analytical results of Oxytetracycline and Ampicillin residues (Ug/Kg) in samples of local and imported frozen broiler meat and giblets (n=20).
S.E*
-Standard error of mean ++-t-test indicated high significant differences (P<0.01)
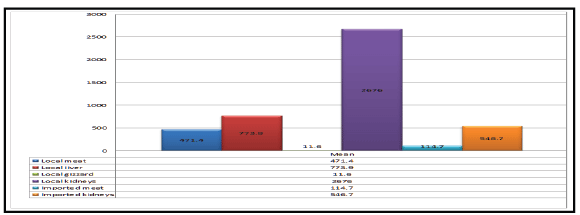
Figure 1: Mean values of oxytetracycline concentrations (ug/kg) in the samples of local and imported frozen broiler meat and Giblets.
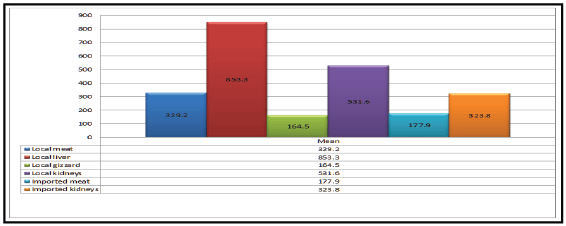
Figure 2: Mean values of ampicillin concentrations (ug/kg) in the samples of local and imported chicken meat and giblets.
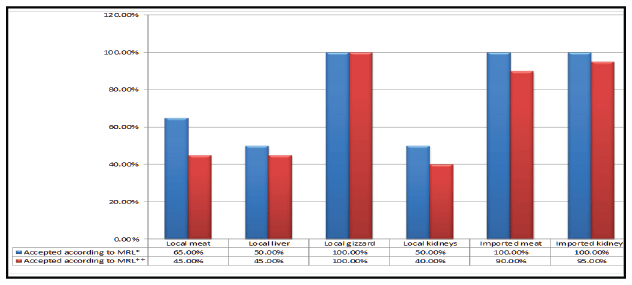
Figure 3: Acceptability of the examined samples of local and imported frozen broiler meat and giblets according to their contents of oxytetracycline residues.
(
*
MRL according to US code of federal regulations, 2003, FAO/WHO, EOSQC, 2008 and Codex Alimentarius Commission, 2012 and **MRL according to EC, 2010)
Otherwise, the ampicillin residues were detected in 50%, 55%, 35%,
50%, 35% and 45% of the examined samples of local broiler meat, liver,
gizzard, kidneys, imported broiler meat and kidneys with mean value of
329.2 ± 20.7, 853.3 ± 65.1, 164.5 ± 9.8, 531.6 ± 37.4, 177.9 ± 15.4 and 323.8
± 26.6, respectively. The incidence of ampicillin residues in examined
samples substantiated what have been recorded by Verdon et al. [21] who
used HPLC method to determined ampicillin residues in kidney, liver and
muscle tissues and the limit of detection was approximately 3-11 ug/kg and
the limit of quantitation was evaluated down to 25ug/kg.
Acceptability of the examined samples of local and imported broiler
meat and giblets based on their levels of oxytetracycline residues was shown
in figure 3. Accurately, 65%, 50%, 50% and 100% of the examined samples
of local broiler meat, liver, kidneys and gizzard were accepted, respectively.
While all examined samples of imported meat and kidneys were accepted
where they did not exceed the permissible limits. This according to MRL of
oxytetracycline (200 ug/kg for muscle, 600 ug/kg for liver and gizzard and
1200 ug/kg for kidney) which stipulated by US code of federal regulations
[22], FAO/WHO [23], Egyptian Organization of Standardization and
Quality Control “EOSQC” No. 3692 [10] and Codex Alimentarius
Commission [12]. Otherwise, according to MRL of oxytetracycline
residues (100 ug/kg for muscle, 300 ug/kg for liver and gizzard and 600 ug/
kg for kidney) which estimated by FAO/WHO (1998) [24] and European
community (EC) [25], the accepted samples of local broiler meat, liver,
kidneys and gizzard based on their levels of oxytetracycline residues were
45%, 45%, 40% and 100%. The accepted samples of imported broiler meat
and kidneys based on their levels of oxytetracycline residues were 90%
and 95%, respectively figure 3. Oxytetracycline (OTC) residues could be
detected with different percentage in samples of chicken tissues by AlGhamdi
et al. [26] who detected that 87% and 100% of poultry muscle and
liver samples had OTC residues respectively.
Acceptability of the examined samples of local broiler meat and giblets
based on their levels of ampicillin residues was shown figure 4. Accurately,
50%, 45%, 70%, 50%, 70% and 55% of the examined samples of local
broiler meat, liver, gizzard, kidneys, imported broiler meat and kidneys
were accepted, respectively. This according to MRL of ampicillin (50 ug/kg
for muscle, liver, gizzard and kidney) recorded by FAO/WHO (1998) [24],
Egyptian Organization of Standardization and Quality Control “EOSQC”
No. 3692 (2008) [10], European community (EC) (2010) [25] and Codex
Alimentarius Commission (2012) [12]. Also, ampicillin residues could be
detected with different percentage in tissue samples by Darwish et al.
[27] at 18%.
The obtained results showed that the highest incidence of oxytetracycline
and ampicillin residues was recorded in broiler liver. These results are
consistent with those reported by Pavlov et al. [28] who mentioned that
the highest residue concentrations were observed in liver and kidney and
residue concentrations in skin fat, abdominal fat and muscle were very low.
Difference associated with the examined samples of local and imported
broiler meat and giblets were highly significant (P<0.01) as a results of their
contents of oxytetracycline and ampicillin residues as shown in table 2.
Results achieved in figure 5 declared the effect of different cooking
methods (boiling, frying and grilling) on oxytetracycline (OTC) and
ampicillin residues in the examined chicken meat samples. Before boiling,
frying and grilling the concentration of OTC residues in chicken meat
were with mean value of 446.16 ug/kg. While, the reduction % in the
concentrations of OTC residues was with mean value of 84.52%, 93.62%
and 96.58% after boiling, frying and grilling, respectively.
Otherwise, the concentrations of ampicillin residues in chicken meat
were with mean value of 253.7 ug/kg before boiling, frying and grilling.
While, the reduction % in the concentrations of ampicillin residues was
with mean value of 81.22%, 90.54% and 94.5% after boiling, frying and
grilling, respectively. From these results, it was concluded that cooking
methods (boiling, frying and grilling) have positive effects on the
oxytetracycline and ampicillin residues in total and partial degrading of
these residues. Thus, application of heat treatment like boiling, roasting,
frying and autoclaving may lead to destroying of all drug residues [29].
Therefore, use of proper cooking processes that have a higher temperature
and longer time can lead to the most reduction in antibiotics residues in
foodstuff and it can provide an additional margin of safety for consumers
[30]. Thus, good cooking was used for removal of great part of the antibiotic
residues such as oxytetracyclin and ampicillin [14].
Conclusions and Recommendations
The obtained results allow to conclude that the examined samples
of local and frozen imported broilers giblets and tissues proved to be
contaminated with residues of oxytetracycline and ampicillin especially
in the liver samples followed by kidney, muscle and gizzard, thus may
constitute health hazards in both animals and human. Furthermore, the
application of different cooking methods (boiling, frying and grilling)
have a positive effects on the residues of oxytetracycline and ampicillin in
degrading the concentrations of residues in such examined samples.
Therefore, to improve the hygienic quality of poultry meat and to
safeguard consumers from being adversely affected from the antibiotic
residues in such meat the ante-mortem examination should be done in
abattoirs to exclude the diseased poultry and suspected samples should
be tested for residues by using easy, simple, sensitive and rapid method
as HPLC test, active surveillance programs to monitor drug residues in
food should be established and Heat treatment of meat should be done to
inactivate antibiotic contaminants in feed stuffs.
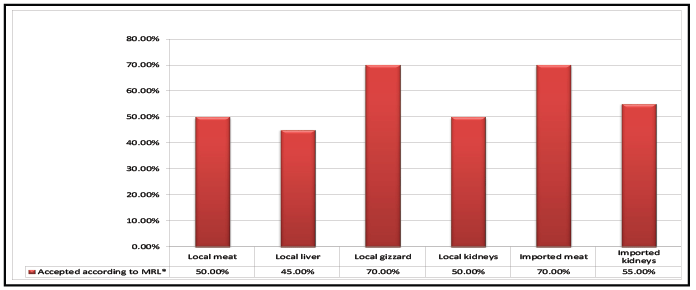
Figure 4: Acceptability of the examined samples of local and imported frozen broiler meat and giblets according to their contents of ampicillin residues.
(*
MRL according to US code of federal regulations, 2003, FAO/WHO, EOSQC, 2008, EC, 2010 and Codex Alimentarius Commission, 2012)
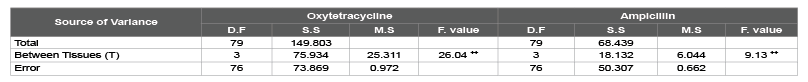
Table 2: Analysis of Variance (ANOVA) oxytetracycline and ampicillin residues in the samples of local broiler meat and giblets.
++-t-test indicated high significant differences (P<0.01)
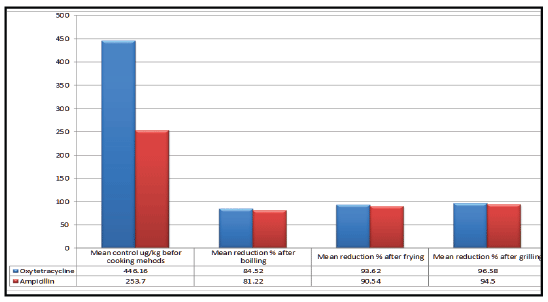
Figure 5:The effect of different cooking methods (boiling, frying and grilling) on oxytetracycline and ampicillin residues in chicken meat (Reduction %).








