
Full Text
LORETA Z-score Neurofeedback in Patients with Medically Refractory Epilepsy
Lauren C Frey 1 J Lucas Koberda 21Epilepsy Monitoring Unit, University of Colorado Hospital University of Colorado, Anschutz Medical Campus, USA
2Tallahassee NeuroBalance Center, Tallahassee, FL, USA
*Corresponding author: Lauren C. Frey, M.D., Associate Professor of Neurology, Director,
Epilepsy Monitoring Unit, University of Colorado Hospital University of Colorado, Anschutz
Medical Campus, USA, E-mail: Lauren.Frey@ucdenver.edu
Article Information
Article Type: Research Article
Citation: Frey LC and Koberda JL (2015) LORETA Z-score Neurofeedback in Patients with Medically Refractory Epilepsy. J Neurol Neurobiol, Volume1.1: http://dx.doi.org/10.16966/2379-7150.102
Copyright: © 2015 Frey LC, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Publication history:
Abstract
Rationale: Published studies suggest that augmentation of the sensorimotor rhythm (SMR), a commonly-used neurofeedback protocol for patients with epilepsy, can be an effective means of reducing seizure frequency, even in patients with medically-refractory seizures. However, SMR protocols are limited to training a few frequency bands over sensorimotor cortex. Newer neurofeedback technology allows for the selection of multiple frequency bands in multiple head regions for training purposes and thus allows for training of neural networks.Functional MRI studies have shown abnormal connectivity within the default mode network (DMN) in patients with both focal-onset and primary generalized epilepsy syndromes. The DMN has also been shown to have altered activity concurrent with interictalepileptiform discharges. The effectiveness of newer neurofeedback techniques in reducing seizure frequency for patients with medically-refractory seizures has not yet been established. This case series explores the potential effectiveness of using LORETA z-score training within the DMN in reducing seizure frequency in patients with medically-refractory seizures.
Methods: The records for all consecutive patients seen in the Neurofeedback Clinic at a single academic medical center over a one year period (n=6) were retrospectively reviewed. All patients had medically-refractory epilepsy and were either not candidates for epilepsy surgery (based on consensus decision of the center’s faculty) or had refused to consider surgery for personal reasons. Data on patient demographics, duration of epilepsy prior to training, seizure types and frequencies, antiepileptic drugs (AEDs), psychiatric and medical comorbidities, imaging results, neurophysiological results, and the duration of neurofeedback training were abstracted and analyzed. Patient-reported seizure frequency was also analyzed.
Results: 125 total training sessions were reviewed. Mean patient age was 33 +/- 6.1 years with mean duration of epilepsy prior to training of 17.2 +/- 3.2 years. Five out of six patients had focal onset epilepsy. None of the patients had a structural lesion on MRI that correlated with their seizure focus. Five out of 6 patients had a history of comorbid mood disorder. No patient had ever been seizure free for more than 1 year. Patients had been trained using LORETA z-score training within the DMN for an average of 20.8 +/- 5.2weeks (1-2 sessions per weekfor 20-30 minutes per session) at the time of analysis. Five out of 6 patients trained had a subjective reduction in reported weekly seizure frequency after LORETA z-score neurofeedback training began.
Conclusions: In this small case series, DMN training using LORETA z-score neurofeedback techniques resulted in subjective improvement in seizure frequency from reported baseline for five out of the six patients in this series.Larger studies are needed to more definitively assess the effectiveness of these techniques for reducing seizure frequency in patients with medically-refractory seizures who are not, for either medical or personal reasons, candidates for surgical intervention.
Keywords
LORETA; Z-score neurofeedback; Epilepsy; Seizures; EEG; QEEG
Introduction
Neurofeedback training is designed to collect, analyze and “feedback” information about an individual’s EEG signals so that the individual can learn to modify their brain activity. Individual therapeutic training goals are based upon significant abnormalities in a baseline quantitative EEG (QEEG). Repeated exercise of the brain pathways needed to reach feedback goals stimulates synaptic and network plasticity, changing brain functioning over the long-term, sometimes permanently [1]. Published studies suggest that augmentation of the sensorimotor rhythm (SMR, a 12-15Hz maximal rhythm produced over primary motor and sensory brain regions), a commonly-used neurofeedback protocol for patients with epilepsy, is thought to act by changing thalamocortical regulatory systems and increasing cortical excitation thresholds[1-3]. As such, SMR neurofeedback augmentation can be an effective means of reducing seizure frequency in patients with drug-refractory seizures [1-4]. However, SMR augmentation protocols are necessarily limited to training a limited frequency band over sensorimotor cortex. Newer neurofeedback technology allows for the selection of multiple frequency bands in multiple head regions for training purposes and thus can be used for training whole neural networks.
The default mode network (DMN) is a spatially-distributed network of brain areas (including areas such as the posterior cingulate cortex, the medial prefrontal cortex, the precuneus and lateral parietal cortex) that is most active during conscious rest and less active during cognitive tasks [5]. Functional MRI studies have shown abnormal connectivity within the DMN in patients with both focal-onset and primary generalized epilepsy syndromes [6-11] and decreased DMN activity with interictalepileptiform discharges [12,13]. The effectiveness of newer neurofeedback techniques in reducing seizure frequency for patients with drug-refractory seizures has not yet been established [14]. This case series explores the potential effectiveness of using LORETA Z-score training within the DMN for reducing seizure frequency in patients with drug-refractory seizures.
| Brodmann Area | Bands Trained |
| 2L | T,A,A1 |
| 2R | T,A,A1 |
| 7L | T,A,A1 |
| 7R | T,A,A1 |
| 10L | T,A,B,A1,A2,B1 |
| 10R | T,A,B,A1,A2,B1 |
| 11L | T,A,A1 |
| 11R | T,A,A1 |
| 19L | T |
| 19R | T |
| 29L | D,T,A,A1 |
| 29R | T,A,A1 |
| 30L | D,T,A,A1 |
| 30R | T,A,A1 |
| 31L | T,A,A1 |
| 31R | T,A,A1 |
| 35L | D,T,A,A1 |
| 35R | T |
| 39L | T,A,A1 |
| 39R | T |
| 40L | T,A,A1 |
| 40R | T,A,A1 |
N |
6 Patients |
Total Number of Training sessions studies |
125 Sessions |
Mean Number of Completed sessions per patient |
20.8+/-5.2 |
Gender |
1male: 5 female |
Mean Patient Age |
33+/-6.1 years |
Mean duration of epilepsy prior to starting training |
17.2+/-3.2 years |
Mean number of antiseizure drugs |
2.3+/-0.4 |
Focal onset epilepsy syndrome |
5 or 6 patients |
Structural lesion on MRI |
0 of 6 patients |
History of comorbid mood disorder? |
5 or 6 patients |
Methods
The records for all consecutive patients seen in the Neurofeedback Clinic at a single academic medical center between November 1, 2013 and November 7, 2014 (n=6) were retrospectively reviewed. All patients had medically-refractory epilepsy and were either not candidates for epilepsy surgery based on consensus decision of the center’s faculty or had refused to consider surgery for personal reasons. Medical refractoriness was defined as having failed to achieve seizure control with two or more anti-seizure drugs [15]. All consensus decisions about patient eligibility for epilepsy surgery were made during a multidisciplinary case conference discussion that included our center’s epileptologists, epilepsy-trained neurosurgeons, neuroradiologists and neuropsychologists. Decisions were based upon the results of long-term video-EEG monitoring, multimodality brain imaging, Wada testing (if applicable) and neuropsychological testing.
For this study, data on patient demographics, epilepsy histories and ancillary testing results were abstracted and analyzed. Patient-reported seizure frequency was recorded prior to each training session. All patients had baseline QEEG studies recorded before each training session. Patients were trained using LORETA Z-score training protocols (NF-2 Neuroguide software, Applied Neuroscience, Inc., Seminole, FL/ DeymedTru-Scan 24 amplifier, Deymed Diagnostic, Colorado Springs, CO) within the DMN for 1-2 sessions per week for 20-30 minutes per session. The protocolspecific areas and frequencies targeted within the DMN were individually designed for each patient selected based on a baseline QEEG study obtained prior to beginning neurofeedback training. A sample protocol from one patient is included in Table 1.
Results
A total of 6 patients were included in this study with a total of 125 training sessions analyzed. Summary information about patient gender, age, epilepsy duration, antiseizure drugs, epilepsy syndromes and brain MRI findings are included in Table 2.
All patients experienced some change in their baseline QEEG with DMN training, suggesting that the LORETA Z-score neurofeedback training was, in fact, altering the patient’s underlying physiology. Two examples of these QEEG changes are included in Figure 1. QEEG analysis from Patient 1 after DMN LORETA Z-score neurofeedback training (B) demonstrated changes in distribution of excess theta power (training target) and improvements in coherence (not a direct training target in this case), when compared to an early QEEG analysis (A). QEEG analysis from Patient 4 after DMN LORETA Z-score neurofeedback training (D) demonstrated improvements in high beta excess (training target) and coherence (not a direct training target in this case), compared to an earlier QEEG analysis (C).
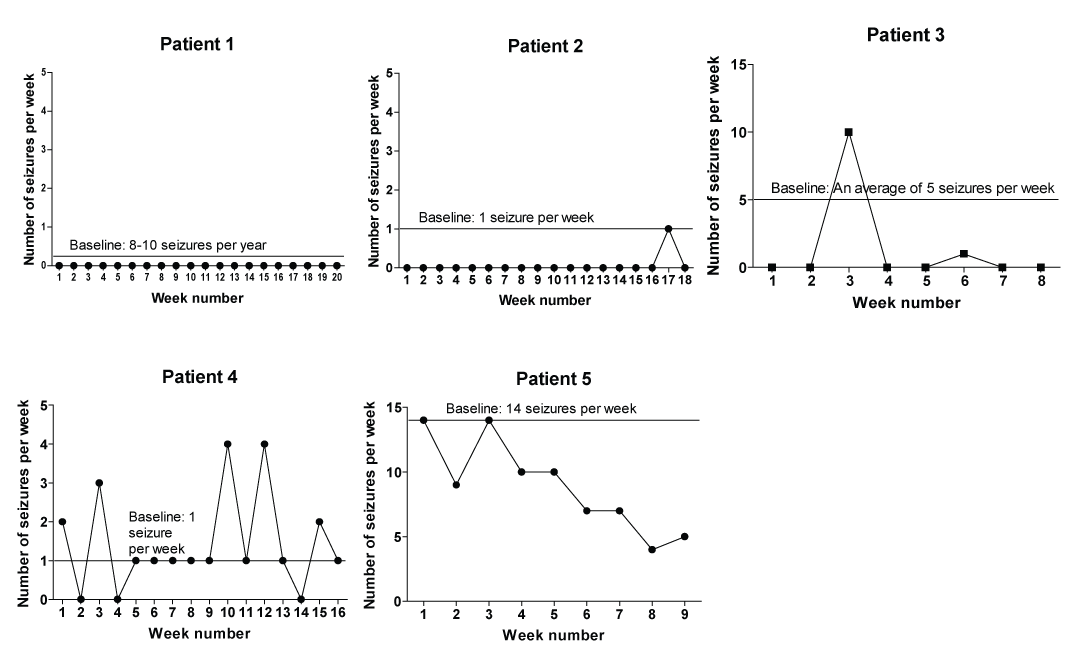
In addition to the changes in their QEEGs, five out of the six patients reported a reduction in weekly seizure frequency after LORETA Z-score neurofeedback training began, as shown in Figure 2. In this figure, weekly recorded seizure frequencies are plotted with respect to the patient’s baseline reported seizure frequency. There were no clear associations between the occurrence of neurofeedback training-related reductions in seizure frequency and patient demographic or clinical epilepsy features.
Conclusions
In this small case series, DMN training using LORETA Z-score neurofeedback techniques resulted in improvement in seizure frequency from reported baseline for five out of the six patients in this series. Published studies have documented abnormal connectivity within the DMN in epilepsy patients [6-11]and decreased DMN activity with interictalepileptiform discharges [12,13], supporting the choice of the DMN as a neurofeedback training target. However, our series of patients did not respond equally or fully (in terms of seizure control) to the DMNbased training protocols, suggesting that further study is certainly needed.
We also found that all of the patients in the series demonstrated change in their EEG spectra, as documented on subsequent QEEG studies. Although we cannot draw a clear associative relationship between these changes and training-related changes in seizure frequency, the presence of changes on QEEG suggests that the power and connectivity of brain regions within the DMN can be altered by LORETA Z-score neurofeedback training.

The main limitation of this study is that we relied on self-report of the number of seizures a patient experienced each week during training and did not have a method for confirming these numbers. However, many of these patients used a web- or phone-based seizure record and provided counts based on their daily counts within these records. For those patients who did not use a web- or phone-based seizure record, the fact that we asked them to provide seizure counts on a weekly basis may have helped mitigate any inaccuracies that may come from difficulty in remembering seizures.
In conclusion, LORETA Z-score neurofeedback training may represent a useful non-pharmacological intervention for patients with medicallyrefractory epilepsy who are not, for either medical or personal reasons, candidates for surgical intervention. Larger studies are needed to more definitively assess the effectiveness of these techniques for reducing seizure frequency in these patients. In addition, tracking the neurophysiological shifts in patient’s QEEG maps will be important to try to correlate choice of training protocol, changes seen in QEEG and seizure outcomes in LORETA Z-score neurofeedback-trained patients.
References
- Sterman MB, T Egner (2006) Foundation and practice of neurofeedback for the treatment of epilepsy. Appl Psychophysiol Biofeedback 31: 21-35.[Ref.]
- Sterman MB (2000) Basic concepts and clinical findings in the treatment of seizure disorders with EEG operant conditioning. Clin Electroencephalogr 31: 45-55. [Ref.]
- Sterman MB Bowersox SS (1981) Sensorimotor electroencephalogram rhythmic activity: A functional gate mechanism. Sleep 4: 408-422.[Ref.]
- Tan G, Thornby J, Hammond DC, Strehl U, Canady B, et al. (2009) Meta-analysis of EEG biofeedback in treating epilepsy. Clin EEG Neurosci 40: 1-7. [Ref.]
- Mantini D, Vanduffel W (2013) Emerging Roles of the Brain’s Default Network. Neuroscientist 19: 76-87.[Ref.]
- Zhang Z, Lu G, Zhong Y, Tan Q, Liao W, et al. (2010) Altered spontaneous neuronal activity of the default-mode network in mesial temporal lobe epilepsy. Brain Res 1323: 152-160.[Ref.]
- Liao W, Zhang Z, Pan Z, Mantini D, Ding J, et al. (2011) Default mode network abnormalities in mesial temporal lobe epilepsy: A study combining fMRI and DTI. Hum Brain Mapp 32: 883-895.[Ref.]
- Song M, Du H, Wu N, Hou B, Wu G, et al. (2011) Impaired resting-state functional integrations within default mode network of generalized tonic-clonic seizures epilepsy. PLoS One 6: 1-6.[Ref.]
- Luo C, Li Q, Lai Y, Xia Y, Qin Y, et al. (2011) Altered functional connectivity in default mode network in absence epilepsy: A resting-state fMRI study. Hum Brain Mapp 32: 438-449.[Ref.]
- McGill ML, Devinsky O, Kelly C, Milham M, Castellanos FX, et al. (2012) Default mode network abnormalities in idiopathic generalized epilepsy. Epilepsy Behav 23: 353-359.[Ref.]
- Danielson NB, Guo JN, Blumenfeld H (2011) The default mode network and altered consciousness in epilepsy. Behav Neurol 24: 55-65.[Ref.]
- Laufs H, Hamandi K, Salek-Haddadi A, Kleinschmidt AK, Duncan JS, et al. (2007) Temporal lobe interictal epileptic discharges affect cerebral activity in “default mode” brain regions. Hum Brain Mapp 28:1023-1032.[Ref.]
- Laufs H, Lengler U, Hamandi K, Kleinschmidt AK, Krakow K (2006) Linking generalized spike-and-wave discharges and resting state brain activity by using EEG/MRI in a patient with absence seizures. Epilepsia 47: 444-448.[Ref.]
- Koberda JL, Bienkiewicz A, Koberda L, Koberda P, Moses A (2013) LORETA z-score neurofeedback as a potential application in epilepsy. in International Society for Neurofeedback and Research. Dallas, TX.[Ref.]
- Berg AT, Vickrey BG, Testa FM, Levy SR, Shinnar S, et al. (2006) How long does it take for epilepsy to become intractable? A prospective investigation. Ann Neurol 60: 73-79.[Ref.]
Download Provisional pdf here
SCI FORSCHEN JOURNALS
All Sci Forschen Journals are Open Access
