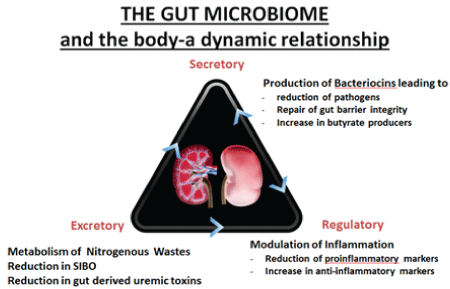
Figure 1: Postulated modes of action by which kidney health product can modulate the gut microbiome and reduce serum levels of uremic toxins.


N Ranganathan1* U Vyas1 K Hanlon1 P Ranganathan1 A Irvin1 A Weinberg2 EA Friedman3
1Kibow Biotech Inc., Newtown Square, PA, USA*Corresponding author: Natarajan Ranganathan, PhD, Kibow Biotech Inc., 4781 West Chester Pike, Newtown Square, Pennsylvania -19073, USA, Tel: +1-610-353-5130; Fax: 610-353-5110; E-mail: rangan@kibowbiotech.com
Background: Based on the concept of “Enteric Dialysis®” Kibow Biotech’s proprietary patented kidney health supplement, has proven helpful in many suffering from CKD, for healthy kidney function by gut microbiome modulation. Available since 2010, it is continually being studied to assess its effectiveness. This survey was to evaluate GFR changes as measured in mL/min per 1.73 m2 , and quality of life (QoL) improvements in customers taking this synbiotic dietary supplement along with their standard care of therapy.
Methods: A survey was distributed to 600 customers taking this product. Questions included GFR before taking this supplement, and at their most recent doctor’s visit, age, race, ethnicity, and QoL. GFR data and QoL was analyzed statistically. 214 (35%) survey responses were received.
Results: The average survey participant was 69 years and using this proprietary supplement for 2.05 years. 150 surveys contained complete information, including GFR. Baseline GFR was 4 to 100 with an average of 29. The most recent GFR varied from 5 to 102. The highest impact on GFR was an increase of 65, and the largest decrease in GFR was -43. The average change in GFR for a survey participant was an increase of 3.55 mL/min/1.73 m2 . 88% of survey participants reported this product improved their QoL.
Conclusion: CKD is generally recognized as a degenerative process. With over 4,000 customers using this pro/prebiotic patented dietary supplement we sought feedback from 15% to assess its impact over an average use of 2.05 years. The longest usage of the product was 7 years, the shortest-6 months. With the ability to stabilize and improve GFR, it may be possible to delay all stages of kidney failure progression. Improved QoL in 88% of participants certainly signifies the advantages of using this patented supplement in patients with compromised renal function worldwide.
Quality of Life; Probiotics; Kidney Function; GFR; Prebiotics; Dietary Supplement; Synbiotic; Chronic Kidney Disease *-This product is called Renadyl™
Chronic Kidney Disease (CKD) refers to a wide range of conditions which damage the kidneys, and reduce their efficiency to filter the blood [1]. CKD is a chronic condition, meaning kidney function continues to decline over time, and the disease generally cannot be prevented or cured by medication/treatment [2]. Recently, increased awareness has come to the prevalence of CKD in the United States and worldwide, as an estimated 30 million American adults suffer from CKD; roughly 15% of the US population. In addition, 48% of those with severely reduced kidney function not on dialysis are unaware of having CKD [3]. CKD is a disease segmented into different stages, I-V, and each stage represents a decreased level of kidney function measured by Glomerular Filtration Rate (GFR). A GFR over 90mL/min/ 1.73 m2 , is considered to be normal [4], however risk factors such as obesity, diabetes, high blood pressure, genetics, old age, and ethnicity increases the risk [5]. Roughly 25% of adults with diabetes in the United States have CKD [6]. Increases in blood pressure increase the rate of decline in kidney function by damaging nephrons and glomeruli [7].
Roughly 70% of Americans aged 20 and older are overweight, of the 70% nearly 33% are considered obese [8]. In children, 17% of children aged 6-19 are obese, and 10% of 2-5 year olds [9]. Following linear time trends suggest 51% of the American population will be obese by 2030; however a model conducted by Finkelstein et al. estimates by 2030 only 42% of the population will be obese with roughly 11% severely obese [10]. As the number of Americans with obesity continues to rise, the number of people with hypertension and diabetes is expected to increase as well. The Framingham Heart Study estimated that being overweight and obese attributed for approximately 26% of cases of hypertension in men, and 28% in women [11]. Roughly 90% of people living with type 2 diabetes are overweight or obese [12].
Current treatment strategies center on delaying the progression of CKD. Once CKD has been diagnosed and a prognosis has been given, nephrologists typically monitor blood pressure. Nephrologists will prescribe various medications such as angiotensin-converting enzyme (ACE) inhibitors, angiotensin receptor blockade (ARBs) and diuretics, as well as make alterations in the patient’s diet such as a reduction in sodium, potassium, and protein intake [13]. The recommended protein intake for CKD patients with or without diabetes and a GFR below 30 ml/min/1.73 m2 is 0.8 g/kg/day [14]. In adults with CKD at risk of progression, protein intake over 1.3 g/kg/ day should be avoided [15]. Clinicians also use a multifactorial intervention strategy for glycemic control, promoting the use of ACE inhibitors, or ARBs, statins, and antiplatelet therapy wherever deemed necessary [16].
Based on the Kidney Disease Improving Global Outcomes (KDIGO) updated 2013 guidelines the National Kidney Foundation-Kidney Disease Outcomes Quality Initiative (NKF_KDOQI) wrote a commentary for practitioners in the United States, to help them interpret KDIGO guidelines and revise the 2002 guideline. This revision includes the management of progression and associated complications of CKD [17]. Prevention of CKD progression includes reducing salt and protein intake, glycemic control for CKD patients with diabetes, to undertake physical activity which is tolerable and compatible with cardiovascular health, smoking cessation, maintain and achieve a healthy weight among others. Other complications associated with CKD which is discussed in this revision includes cardiovascular disease which is highly prevalent in CKD patients, and medications for patients with CKD who have cardiovascular complications. A 10 year follow up study conducted by Tsai and his team in 4,600 individuals with CKD, particularly those in stage IV and V found that the median average decline rate of eGFR was 4.42 mL/min/1.73 m² per year for the group identified as rapidly progressing towards ESRD [18].
Probiotics are defined as non-pathogenic microorganisms which confer a health benefit upon the host consuming them [19]. The most common use for probiotics is in gastrointestinal (GI) applications such as digestive, gut and immune health, specifically for traveler’s diarrhea and pouchitis [20]. Recently more research is being conducted in the use for other indications such as irritable bowel syndrome (IBS), ulcerative colitis (UC) and from our extensive work towards chronic kidney diseases (CKD).
It is estimated that the gut microbiome contains roughly 38 to 100 trillion bacterial cells [21,22]. The condition known as dysbiosis, which refers to an imbalance in the composition of the gut microbiome, has been linked to a wide range of diseases such as obesity, diabetes, cancer, cardiovascular, autism, allergy [23]. As scientists and researchers continue to uncover the metabolic impact, the gut microbiome plays in health and disease; there is more evidence especially on the role the gut microbiome has to play in CKD [24].
At the intestinal epithelial barrier, the gut microbiome and the bloodstream interact with one another creating a passive diffusion. This is the location where toxins and other metabolites passively diffuse into the gut. Conversely, toxins and other metabolites formed in the gastrointestinal tract by the pathogenic gut bacteria are able to passively diffuse into the blood stream [25]. These metabolites like the lipopolysaccharides of the bacteria bring about inflammation [26]. Also, increased intestinal concentration of uremic toxins is associated with bacterial overgrowth and microbial dysbiosis of pathogenic microbes [25]. In patients on hemodialysis, it was found that lower numbers of bifidobacteria and more Clostridium perfringens were found in their gut microbiome. The study by Hida’s team also showed that aerobic species of bacteria such as Enterobacteria, and Enterococci were approximately 100 times more prevalent [27]. Patients with CKD also have an increased growth of pathogenic bacteria in their small intestine which is termed as Small Bowel Bacterial Overgrowth (SBBO) or Small Intestinal Bacterial Overgrowth (SIBO) as observed by Simenhoff and his team [28]. Operational Taxonomic Units (OTUs) are primarily used to classify groups of closely related individuals, i.e. members of the same species. In microbiology, OTUs are used to identify species of microorganisms in Metagenomics’ studies. Metagenomics’ analyses of the gut microbiome are done using 16S rRNA as a target to separate the various OTUs in a given sample [29].
Enteric Dialysis® as a concept originated from Dr. Eli A. Friedman’s article, “Can the Bowel Substitute for the Kidney in Advanced Renal Failure?” [30] Enteric Dialysis® is a technology that modulates the gut microbiome in chronic kidney disease patients. By supplementing the gut microbiome with probiotic bacteria, it is possible to metabolize the nitrogenous waste products and other toxins which diffuse into the gut, and thus lower levels of inflammation [31]. These pathogenic bacteria produce harmful middle-molecules and generate additional toxins in micro molar concentrations, which significantly impact the patient’s quality of life [32,33]. Enteric Dialysis® involves the process of introducing highly specific strains of probiotic bacteria to metabolize various uremic toxins which accumulate as a result of decreased kidney function. It also combats the pathogenic bacteria through naturally secreted bacteriocins or antimicrobial peptides which possess antibiotic like properties leading to less production of harmful middlemolecules and metabolites and thus helps in turn to improve overall quality of life [34].
The patented proprietary probiotic and prebiotic formulation contains three highly specifically screened, tested and chosen strains of probiotic microbes Streptococcus thermophilus (KB19), Lactobacillus acidophilus (KB27), and Bifidobacterium longum (KB31). Each strain of microbe was selected for their ability to metabolize various specific uremic toxins such as urea, uric acid, creatinine, phenols, indoles, cresols, methylamine, and trimethylamine. Past clinical trials have been conducted using this product towards reducing several uremic toxins and as well to evaluate QoL parameters [31,34-36]. However a survey ascertaining the effect of this product supplement on changes in GFR has not been completed to date. In 2014 the NKF/FDA suggested primary endpoint for a CKD treatment/therapy is reducing the decline in GFR by 30% (40% is preferred) [37]. The present survey was sent out to see the effect of this product on GFR and also to validate the need to test this supplement in a large-scale double-blind, placebo-controlled study.
This survey was the third consecutive biennial survey given to assess the impact of Enteric Dialysis via this synbiotic supplement on improving quality of life (QoL), as well as evaluate the GFR function among the survey respondents in various CKD stages. In light of the recent NKF/FDA preferred primary endpoint on reduction in the decline of GFR by 40% [36], there was interest in assessing the impact that Enteric Dialysis has on kidney function, and to determine if this kidney health supplement could have a positive drug-like efficacy in assessing the progression of GFR decline. A simple survey questionnaire (Table 1) was sent to 600 randomly chosen customers who have been taking the kidney health supplement from our database of customers.
| A | Demographics |
| Age | |
| Sex | |
| Ethnicity | |
| B | Co morbidities |
| Hypertension (high blood pressure) | |
| Heart Disease | |
| Diabetes | |
| Others | |
| C | Product Related Questions |
| When did you begin taking this proprietary product? | |
| What was your GFR when you began taking this product? | |
| What is your GFR now? | |
| Has this supplement improved your quality of life(less stress, Greater productivity, higher activity level, better appetite etc)? Y/N | |
| D | Kindly provide a brief and candid testimonial about your experience with this kidney health supplement thus far |
Table 1: Survey Questionnaire
Of the 600 surveys sent, 214 responses (35.6%) were received. Unlike the first two surveys which were carried out in 2013 and 2015, for this survey GFR data was specifically requested on a voluntary self-reported basis. The previous two surveys did not ascertain GFR information in compliance with HIPPA regulations. GFR was initially compared at two time points, i.e. baseline and then at the time the survey was reported. Since follow-up differed for each patient, average change in GFR per year was compared in our statistical analysis. A mixed modeling procedure using PROC MIXED (SAS) was incorporated to model changes according to the various years of follow-up, and to discern whether there were differences in GFR over time. This takes into consideration on the repeated ANOVA measurements per patient, and then estimated whether GFR differed among the various years of follow-up.
Since repeated measurements within patients may be correlated, this procedure allows one to model a “correlation structure”, commonly referred to as a covariance pattern. This method of estimate will allow for estimates of the standard errors of measurement, and therefore more powerful tests. It also allows for estimates at all-time points if data can be assumed to be missing at random (MAR).
There are a number of various covariance structures to consider using. Three of the more common covariance structures include “compound symmetry” (cs), for correlations that are constant for any two points in time, “auto-regressive order one” (ar1), for correlations that are smaller for time points further apart, and “unstructured” (un), which has no mathematical pattern within the covariance matrix. The compound symmetry structure provided the best fit. We were also able to test whether other factors such as gender, age, stages of CKD and other comorbid conditions such as hypertension and diabetes may play a role in GFR changes over time using the mixed method procedure. Chi-square analysis was used to explore whether there was an association between gender and related co-morbid conditions and Quality of Life. Data were analyzed using SAS system software (SAS Institute Inc., Cary, NC).
esults Of the 214 responses received, not all came back completed. There was missing information in many. 206 (96.2%) customers stated their age, 196 (91.5%) gave information about the time they took the product, 150 (70.1%) answered questions on GFR before and after taking the supplement and 200 (93.45%) respondents volunteered information about their sex which was 117 Male and 83 Females. The average age of a survey respondent was 69 years, with a range of 8 to 99. The average GFR1 (baseline GFR) was 30.5 ml/min/1.73 m2 ranging from 4-100 mL/min/1.73 m2 , and the average GFR2 (most recent measured GFR) was 34.0 ml/min/1.73 m2 ranging from 5-106 mL/min/1.73 m2 . The average change in GFR from the beginning of the product use to the respondents most recent doctors visit was an increase of 3.5 ml/min/1.73 m2 . The increase yielded a P-value of .0013, and is statistically significant. The average survey respondent has been using this patented supplement for roughly 3 years (Table 2).
| Variable | N | Mean | Std Dev | Minimum | Maximum |
| gfr1 | 150 | 30.5 | 17.2 | 4.0 | 100.0 |
| gfr2 | 150 | 34.1 | 20. | 5.0 | 106.0 |
| diff | 150 | 3.5 | 13.2 | -40.0 | 65.0 |
| Year change | 141 | 2.9 | 8.4 | -16.0 | 42.2 |
Table 2: Table of Means
Notes: This table displays metrics of the age
Abbreviations: starting GFR (gfr1) current GFR (gfr2), difference in GFR from start to current (diff), and average change in GFR per year (Year change).
200 of the survey respondents indicated a yes or no answer indicating whether or not the product impacted their overall quality of life. 176 (88%) of respondents indicated that it did in fact improve overall quality of life (Table 3) although no scale was assigned in the survey questionnaire.
12% of survey respondents indicated it did not have an effect on overall quality of life. It is worth noting that in the survey this product may have been better at improving quality of life in women than in men. 92% of women reported that this supplement improved overall quality of life whereas 84% of men reported the same. Although this was not found to be statistically significant (p-value of 0.0803), it may be of some significance in endocrine hormonal differences.
| Quality of Life (QoL) by sex | |||
| QoL | Sex | ||
| Frequency | Female(F) | Male(M) | Total |
| No | 6 | 18 | 24 |
| %of M/F | 3 | 9 | 12 |
| %No by M/F | 7.23 | 15.38 | |
| Yes | 77 | 99 | 176 |
| %of M/F | 38.5 | 49.5 | 88 |
| %Yes by M/F | 92.77 | 84.62 | |
| Total | 83 | 117 | 200 |
| Percent M/F | 41.5 | 58.5 | 100 |
| Frequency Missing = 14 | |||
Table 3: Impact of supplement product on Quality of Life
Notes: Product’s impact on survey respondents’ quality of life (QoL). Each answer (yes or no) is broken up by sex. Frequency and percent of group are given in each cell.
CKD is on the rise globally. End Stage Renal Disease (or ESRD) leads either to dialysis or the need for a kidney transplant. Both treatments are extremely expensive with a multitude of side effects. Outcomes are also poor [38]. Dialysis is the primary option for most patients with ESRD due to shortage of transplant kidney availability. Increasing evidence has shown that the human intestinal tract which harbors trillions of microbes and encodes for 3.3 million genes have a huge role to play in human health and almost on all diseases [39,40]. Chronic Kidney Disease (CKD) also involves the gut microbes apart from the chronic nature and pathophysiology of the disease. It is now recognized and known as the Gut-Kidney axis. A large number of clinical studies in CKD progressing to ESRD/ dialysis have shown that renal failure is also associated with an altered microbial/gut microflora. Patients with ESRD were shown to have lower levels of the beneficial bacteria namely Lactobacillus, Bifidobacteria and Prevotella and higher amounts of pathogenic groups like Enterobacteriaceae and Pseudomonadaceae [41,42]. The state of the gut having more pathogenic bacteria as against healthy human gut is termed gutdysbiosis. Multiple studies have shown that kidney failure and gut microflora alteration leads to the build-up of various microbial or so called gut derived toxins. These are the indoxyl sulfate (IS), p-cresyl sulfate (PCS), Trimethylamine-N-oxide (TMAO) among others [43-48]. Uremia thus affects both compositions of the gut derived and metabolism of microbial toxins leading to gut imbalance and gut dysbiosis. The resulting imbalances of the gut microbes and dysbiosis generate many new low levels of uremic toxins, which are more toxic in comparison to urea, uric acid and creatinine. Thus increased formation of toxins due to (1) poor renal clearance of nitrogenous wastes; urea, uric acid and creatinine and (2) gut dysbiosis leading to productions of IS, PCS and TMAO and many other toxic metabolites by gut pathogens could probably lead to rapid progression of the disease conditions towards ESRD.
Medical research to develop drugs to aid the population with CKD is an ongoing field. According to the clinical guidelines given for renal failure, a person is defined as having kidney failure when his or her GFR drops to 15 ml/min/1.73 m3 . This is a CKD stage V also known as ESRD. The only option for the ESRD patient upon this diagnosis is dialysis or a kidney transplant. By this time the patients already have a large number of other comorbid conditions [49,50]. As per the earlier NKF/ USFDA the end point for clinical trials in kidney disease is halving of the glomerular filtration rate (GFR), which is measured as doubling of serum creatinine. This requires a large sample size and a very long follow-up time. Doubling of serum creatinine means decline in the eGFR by approximately 57%. This means that patients in early stages of renal failure are not diagnosed until they reach this stage, which is stage III CKD. In essence all lab reports indicate the value of GFR only if it less than 60ml/1.73 m3 . As a result, in 2014 the National Kidney Foundation (NKF) and USFDA jointly sponsored a workshop for alternate GFR based end points for better assessment of clinical trials to reduce sample size, shorten the duration of trials and also extend the application to all stages of CKD [37]. The new endpoint is now “a confirmed decline in estimated GFR of 30% over 2 to 3 years may be an acceptable surrogate end point. An estimated GFR decline of 40% may be more broadly acceptable than a 30% decline across a wider range of baseline GFRs and patterns of treatment effects on GFR”. Any therapy which can address GFR has a goal to preserve the residual kidney functions, and also try to delay further progression of the disease. Some published alternate treatment options include the use of drugs like alpha keto acids and Kremezin (an activated form of charcoal). Keto acids are highly expensive with a daily dosage of 10-20 grams and Kremezin at 9 grams per day. Kremezin being an activated charcoal can also adsorb some of the drugs which the patient is taking. It also does not remove hydrophilic compounds like urea, which is primarily retained in large quantities in renal failure patients. Studies have demonstrated that urea is carbamylated and this carbamylated form of urea is highly toxic [51].This has also been discussed by our published commentary in the journal of Nephrology and Therapeutics [52]. Very recently at the 2017 American Society of Nephrology kidney week conference in New Orleans held during October 31-November 5, 2017, the department of Health and Human Services (HHS) announced that they would establish a public-private fund called the Kidney Innovation Accelerator to help move new technology forward in treating kidney disease [53]. The reason for this is the lack of new drugs being developed for the management of kidney disease. This fund would be capable of seeding and accelerating not just incremental improvements in treating kidney disease, but will foster real breakthroughs in dialysis and other treatments for kidney disease.
Drugs to improve GFR and treat CKD can delay the progression through different mechanisms; however, they cannot restore the dynamic balance of the dysbiosed gut. Recently it has also been shown that the microbes present in the gut can metabolize drugs [54-57], and maybe render the drug ineffective or produce unwanted metabolites of the drug, thus reducing drug efficacy and possibly creating some adverse and harmful effects too.
As per the US Renal data system report “2017 Annual Data Report Highlights” [58], (A) 14.8 percent of U.S. adults have chronic kidney disease which is 30 million Americans, and with millions of others at risk (B), Medicare spending for beneficiaries who have chronic kidney disease was nearly $100 billion. This includes over $64 billion for CKD and $34 billion for ESRD patients. Thus, it is now the need of the day to focus on reversing the gut dysbiosis-reducing the amount of the pathogenic bacteria in CKD gut, and restoring the gut microbial balance leading to a healthy gut. So along with drug therapy and medications, which is the standard care of therapy alternative methodologies to address the gut related, uremic toxins are indeed highly warranted.
The role of probiotics and prebiotics in health and disease is growing at an exponential rate. The role of the gut microbiome is expanding into every area of human health including CKD. Academic researches by different scientists have shown the ability of certain groups of beneficial bacteria termed “Probiotics” to restore gut balance and reduce some of the gut derived toxins [57-62]. As CKD continues to impact more and more people, novel therapies will need to be developed to improve patient outcomes and reduce treatment costs in all countries.
The product used in this study is a specialized patented and proprietary probiotic and prebiotic (synbiotic) dietary or kidney health supplement developed for CKD patients. It works by different mechanisms in the gut of renal failure patients by way of a unique technology termed “ENTERIC DIALYSIS”. This is depicted in the figure below (Figure 1).

Figure 1: Postulated modes of action by which kidney health product can modulate the gut microbiome and reduce serum levels of uremic toxins.
Three pilot scale clinical trials [31,35,36] and three biennial surveys [34,63] (2017) of customers taking this product showed that these precisely researched and selected probiotic strains in the kidney supplement can indeed benefit the renal failure population by several possible-mechanisms. (A) Removal of nitrogenous wastes by metabolism of uremic toxins urea, uric acid and creatinine resulting in increased growth of beneficial bacteria and reduction in growth of pathogens [25,32,35,64,65]. (B) All three strains produce bacteriocins (antimicrobial molecules) which further inhibits the growth of pathogens [66- 68]. (C) L acidophilus is well documented to reduce levels of cardiovascular toxins TMA and TMAO [64,65]. (D) B longum reduces production of phenols, cresols etc. which get bound to serum proteins and usually not removed by routine dialysis [69]. (E) Modulation of inflammation like CRP reduction which is a holistic biomarker [70,71]. The prebiotic components in this product namely Inulin and Xylooligosaccharide also have various benefits [72-75].There are three ways a prebiotic helps the probiotic bacteria. The prebiotics can: 1) increase probiotic stability during storage and gastrointestinal passage, 2) act as the food, therefore, increase probiotic growth, or 3) indirectly promote growth, by changing the microbiota for better growth conditions, more so according to individual’s own genetic microbiota.
The results of this survey distributed to the customers of this proprietary kidney health supplement, suggest that these guidelines can be further documented in a large-scale, doubleblind, placebo controlled trial (RCT). These results displayed that this supplement has a positive impact on kidney function in this set of survey respondents in various stages of CKD. Although it is subjective data analysis, on an average a 3.5 mL/ min/1.73 m2 increase translates to an 11.6% improvement in GFR (the average GFR at the start of taking the product was 30 mL/min/1.73 m2 ) which is considered CKD stage IV. With such strong documented stabilizations and also improvements in GFR, it is not surprising to see that 88% of survey respondents indicated that their quality of life improved after taking the product. Two prior customer surveys distributed in 2013 [63] and 2015 [34] were mainly focused on ascertaining quality of life data analysis. In 2013, 72% of respondents indicated that this kidney health supplement improved quality of life, 2015 saw 73% of respondents indicating quality of life improvements, and the 2017 survey (which also featured an optimized formulation) has further improved the QoL by an additional 15%. Such results are promising for the kidney health supplement and warrants further validation in a large scale RCT clinical study. This is the first study reporting on further documentation with statistically significant data analysis on the usage of probiotics resulting in stabilization/improvement of GFR in patients with CKD from this 3rd - biennial customer survey.
In addition our previous survey in 2015 indicated there was an improvement in GFR [34]. However, this parameter was not the focus at that time. Since the FDA/NKF guideline mandated the primary endpoints in CKD treatments/therapies is to reduce the decline of GFR by 30% (40% is preferred), we extended this third biennial survey with GFR in mind.
Table 4 and Figure 2 demonstrate the possible impact of this product compared to normal CKD progression, and using any interventional therapy that decreases the progression of CKD by 40% (the preferred NKF/FDA guideline primary end point).
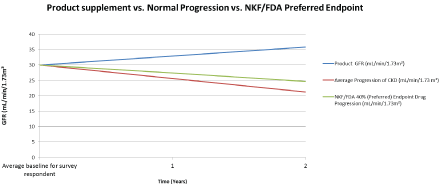
Figure 2: Kidney healthProduct supplementation effects compared to Normal Progression and Preferred NKF/FDA Endpoint.
Notes: A graphical representation of the progression of CKD in someone who is using Kidney health product (projection based off of survey results), normal progression of CKD (Tsai et al. 2017), and using the NKF/FDA preferred endpoint of a 40% reduction in the decline of CKD.
| Year | Product GFR (mL/ min/1.73 m²) |
Average Progression of CKD (mL/ min/1.73 m2) | NKF/FDA 40% (Preferred) Endpoint Drug Progression (mL/ min/1.73 m2) |
| 1 | 30 | 30 | 30 |
| 2 | 32.9 | 25.6 | 27.4 |
| 3 | 35.8 | 21.2 | 24.8 |
Table 4: Product supplement Compared to Normal Progression and Preferred NKF/FDA Endpoint
A period of three years was used (baseline, year 1, and year 2) to coincide with the NKF/FDA guideline. The average survey respondent had a baseline GFR close to 30 mL/min/1.73 m2, for this reason we used 30 as a baseline for all three GFR assessment. The average increase for respondents was 3.5 mL/min/1.73 m2, dividing that by the average time of three years they took the product, gives you an average per year increase in GFR of 2.90 mL/min. The normal progression of CKD based on the 2017 study conducted by Tsai and his group [18], would lead to a decrease in 4.42 mL/min/1.73 m² per year. Using this as the normal progression, the FDA/NKF preferred guideline would reduce the decline in GFR by 40%, so the annual decrease in GFR would be 2.6 mL/min per year. After a baseline (year 1) and two follow-up years, the GFR of a CKD person using this product would be at 32.4 mL/min, a person with normal progression would be at 21.2 mL/min, and a person using a therapy resulting in 40% (preferred NKF/FDA guideline) slower progression would be at 24.8 mL/min. This product user would have a GFR 11.2 mL/min better than someone who is experiencing normal progression of CKD, and this product user would have a GFR 7.8 mL/min better than someone using a theoretical therapy decreasing the decline in GFR by 40%.
Quality of Life (QoL) is another important parameter affecting CKD population. Patients are on long-term medications, dialysis populations are on invasive procedures. Dietary restrictions are also imposed. All of this leads to depression, pain and fatigue thus affecting the patients negatively. Patients with renal failure experience a poor QoL. In one such study 155 patients in CKD stages I to V and 36 hemodialysis patients were studied using the SF-36 questionnaire [76]. They observed a decrease in QoL in all stages of the disease with reduction in physical functioning, depression among others. The concept of health-related quality of life (HRQoL) is now being recognized for patients with CKD. HRQoL is a significant key indicator of how a specific disease condition affects the patient’s life. It is defined as ‘the objective assessment of the impact of disease and its treatment across the physical, psychological and social domains of functioning and well-being [77]. HRQoL is a critical outcome measure for patients with reduced kidney function. HRQoL has been shown to be associated with increased mortality in patients with ESRD [78,79]. Pagels and team in 2012 [80] evaluated HRQoL in 535 patients in various stages of CKD. They observed that all HRQoL deteriorated significantly as CKD progressed. Stage V CKD had the lowest scores. Inflammation and cardiovascular conditions worsened it further. In yet another recent study in 2016 [81], 46,676 community dwellers with CKD were studied. Park and his team observed that CKD stage II and beyond had lower HRQoL scores, which decreased progressively as the stage of CKD increased. Clinical trials with this synbiotic dietary kidney health supplement and our previous two customer surveys showed that patients experienced an improved QoL after taking this product. Since CKD stages are related to declining GFR and progression in CKD stages leads to worsening of the QoL, it is reasonable to assume that GFR and QoL are directly proportional.
Chronic kidney disease is generally recognized as a degenerative process. Studies on the gut microbiome and CKD have shown that there is a gut-kidney connection with a large amount of pathogenic bacteria in the colon of CKD patients which produce the so called gut derived uremic toxins. A strong correlation thus exists between the gut microbial ecosystem and CKD. This synbiotic dietary supplement with a drug like validation shows a promising potential for CKD and ESRD patients on dialysis in maintaining kidney health. Our previous two surveys have shown that this product supplement improves the QoL in patients with CKD. Data from this survey shows 88% of survey respondents indicated improvements in quality of life and also an improvement/stabilization of GFR. While largescale clinical studies are needed to further validate the efficacy of this supplement, this survey can serve as a foundation since we saw a statistically significant improvement/stabilization in the GFR of these survey customers/kidney failure patients who have been taking the product for a minimum of six months. With the ability to stabilize and improve GFR, it may be possible to delay the progression of kidney failure at all stages. Improving quality of life in 84% of participants certainly signifies the advantages of using this dietary supplement in patients with compromised renal function worldwide.
N Ranganathan and P Ranganathan are employees and stockholders of Kibow Biotech, Inc. U Vyas, and A Irvin, are also employees and hold minor stock options in Kibow Biotech, Inc. A Weinberg is a consultant with a small token honorarium payment for his time and valuable efforts on this paper; E A Friedman is the chairman of the Scientific Advisory Board at Kibow Biotech, Inc.
Download Provisional PDF Here
Article Type: RESEARCH ARTICLE
Citation: Ranganathan N, Vyas U, Hanlon K, Ranganathan P, Irvin A, et al. (2018) Improvements in Glomerular Filtration Rate (GFR) in Chronic Kidney Disease (CKD) Patients Using a Commercial Patented and Proprietary Probiotic-Prebiotic Formulation* -3rd Biennial Survey. Int J Nephrol Kidney Fail 4(1): dx.doi.org/10.16966/2380-5498.154
Copyright: © 2017 Ranganathan N, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Publication history:
All Sci Forschen Journals are Open Access