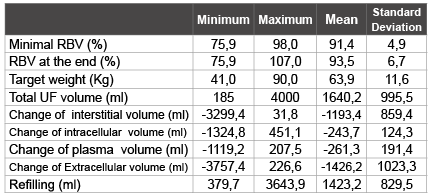
Table 1: Ultrafiltration, Changes in fluid compartments and refilling data

Alayoud A1* Maoujoud O1 Aattif T2 El Kabbaj D2 Benyahia M2 Zemraoui N3
1Service of Dialysis, Military Hospital Agadir, Morocco*Corresponding author: Alayoud Ahmed, Service of Dialysis, Military Hospital Agadir, Morocco, E-mail: a_alayoud@yahoo.fr
Background: The inadequate plasma refilling is the most important factor involved in the pathogenesis of dialysis discomfort. This work explores the determinants factors of vascular refilling and its effects on hemodynamic stability in dialysis.
Methods: Cross-sectional study conducted in 35 hemodialysis patients. Relative blood volume (RBV) was performed by continuous optical measurement of the haematocrit. Hydratation status was measured by bioelectrical impedance spectroscopy.
Results: Mean calculated refilling was 1423 ± 829 ml. The change of the interstitial volume was -1193 ± 859 ml (84% of refilling volume). The change of the intracellular volume was -243 ± 124 ml. There is a significant correlation between the RBV at the end of dialysis and; Delta Prot (post dialysis serum protein-Predialysis serum protein) (r=-0.7, p=0.01) , Delta Natremia (Sodium concentration at the end-Sodium concentration at beginning) (r=0.46, p=0.032), interdialytic weight gain (r=-0.7, p<0.001) , Extracellular water before dialysis (r=-0.53, p=0.001), Total body water before dialysis (r=-0.45, p=0.012), and Ultrafiltration rate (r=-0.85, p=0.0001). The change of systolic blood pressure (ΔSBP=Predialytic SBP- Postdialytic SBP) increased while the RBV decreased at the end of dialysis (r= -0.5, p=0.007). Intradialytic hypotension was more frequent with low RBV.
Conclusion: Several patient-related parameters and technique-related variables affect the plasma refilling, and their control can improve intratreatment hemodynamic stability during dialysis sessions.
Plasma refilling; Dialysis; Hemodynamic stability; Hydratation status
Many factors have been implicated in the pathogenesis of hemodynamic instability during dialysis, including cardiac dysfunction, autonomic dysfunction and the imbalance between the ultrafiltration rate and the plasma refilling rate volume [1-3]. During haemodialysis (HD) the fluid is removed by ultrafiltration from the intravascular compartment, but it naturally derived from the intravascular, interstitial and intracellular volume. This implies a continuous refilling of fluid from the extravascular to the plasma compartments. The plasma refilling rate is the difference between the total fluid loss and plasma volume loss per time unit.
The inadequate plasma refilling is the most important factor involved in the pathogenesis of dialysis discomfort [2]. Therefore, blood volume (BV), interstitial fluid volume and intracellular volume, play an important role in understanding fluid dynamics during HD and continuous registration of plasma refilling with ultrafiltration (UF) is advocated as a tool to maintain an adequate volume of the intravascular compartment to avoid HD hypotension [1,4]. Changes in effective blood volume (EBV) can be measured by radio-isotope dilution techniques, but these methods are not easily applied on a routine basis. Changes in relative blood volume (RBV), however, can be estimated by means of continuous haematocrit (HCT) measurements [3]. In this study we studied the determinants factors of vascular refilling and its effects on hemodynamic stability in dialysis.
35 chronic stables haemodialysis patients treated three times per week from the Military Hospital of Instruction, Mohammed V (Rabat, Morocco)) were recruited to participate in this study after obtaining informed consent. All patients studied were older than 18 years and have intact arteriovenous fistulas. Haemodialysis was performed using Fresenius 5008 therapy system (Fresenius Medical Care,) and high flux (Polyflux 170H, Polyflux 210H, and Polyflux 140H) (Gambro Healthcare) dialyzers. The duration of dialysis treatment was 4hours. The biofeed back control (ultrafiltration control) was deactivated. No sodium modeling was used. Dialysate temperature was kept constant at 37°C. Average blood flow was 300 mL/m . Dialysate flow was set at 500 mL/m. Food and fluid intake was prohibited during the course of dialysis session.
Hydratation status (total body water, Extracellular and Intracellular volume) was measured before dialysis by bioelectrical impedance spectroscopy using the Body Composition Monitor (BCM, Fresenius Medical Care).
During dialysis blood pressures (systolic diastolic and mean), pulse rate was measured at 15 min intervals. Hypotension was defined as a systolic blood pressure <90 mmHg.
Relative blood volume (RBV) was performed by continuous optical measurement of the haematocrit using the Blood Volume Monitor (BVM) device (BVM, FMC). The mathematical formula used to determine RBV at moment t is:
$$RBVt\, = {{EBV\,current} \over {EBV\,starting}} = {{HTC\,starting} \over {HTC\,current}}$$
So blood change during dialysis (ΔVb) is equal to plasma change (ΔVp):
ΔVp=ΔVb=Vb0( RBVt-1)
Blood volume (Vb0) at the beginning of the treatment was estimated anthropometry from the Nadler Method based on the following formulas [5]:
For Males=0.3669 × Height in M3+0.03219 × Weight in kgs+0.6041
For Females=0.3561 × Height in M3+0.03308 × Weight in kgs+0.1833
During haemodialysis the fluid is removed by ultrafiltration (UF) from the intravascular (Vp), interstitial (Vis) and intracellular (Vic) volume.
ΔVT=-UF=ΔVp+ΔVic+ΔVis
The plasma refilling rate is the difference between the total fluid loss and plasma volume loss
Ref=ΔVp+ΔUF=-(ΔVic+ΔVis)
Changes of Vic during dialysis can be modeled, from intracellular volume at the beginning (Vic starting) and the sodium concentration (Na), according to this equation [6]:
$$_\Delta Vic = Vic\,starting\, \times \left( {{{Na\,starting} \over {Na\,current}} - 1} \right)$$
Comparative study was conducted using Student’s paired t-test. Correlation was assessed using Pearson’s coefficient and Spearman coefficient for non parametric variable. A two sided p value 0.05 was considered significant. All statistical analyses were performed with the SPSS version 16 (SPSS Inc., Chicago, IL).
16 women and 17 men with an average age of 50 ± 15 years were enrolled in the study. They had been receiving haemodialysis for 60 ± 50 months. 15% were diabetics. The haematocrit at baseline was 33 ± 4%. Serum protein before dialysis was 63 ± 4g/l.
Mean ultrafiltration volume was 1640 ± 995, with a mean body target weight of 64 ± 11Kg; this represents 2.5% of the total body weight.
For all patients the RBV at the end was 93.5 ± 6.8%. Minimal value of RBV was 91.4 ± 4.9%. For all measurements the average of RBV was 94.8 ± 4.6 % with extremes ranging from 76 % to 108%.
Mean calculated refilling was 1423 ± 829 ml. The change of the interstitial volume was -1193 ± 859 ml (84% of refilling volume). The change of the intracellular volume was -243 ± 124ml; this represents 16 % of the total refilling volume (Table1).

Table 1: Ultrafiltration, Changes in fluid compartments and refilling data
At the end of dialysis sessions, 87% of the fluid removed by ultrafiltration had been refilled from the extra vascular space (13% from intra vascular space).
There is a significant correlation between ΔVic and the ratio of the intracellular water to total body water before dialysis (p=0,03, r=0,48) , and the conductivity (p=0,004, r=0,7).
There is a significant correlation between the RBV at the end of dialysis (RBV final) and; DeltaProt(Post dialysis serum protein-Predialysis serum protein) (r=-0.7, p=0.01) , Delta Natremia (Sodium concentration at the end- Sodium concentration at beginning) (r=0.46, p=0.032), interdialytic weight gain (r=-0.7, p<0.001) , Extracellular water before dialysis (r=- 0.53, p=0.001), total body water before dialysis (r=-0.45, p=0.012), and UF rate (r=-0.85, p=0.0001) . There was not a significant correlation between the final RBV and intracellular water before dialysis (r=-0.19, p=0.278).
The change of the intravascular volume ΔVp was higher in diabetic patients (p=0.04), and in the elderly (p=0,03), Neither there was no impact of gender(p=0.09).
There was a significant correlation between change of RBV and change of heart rate (r=-0.4, p=0.03) (Figure 1).
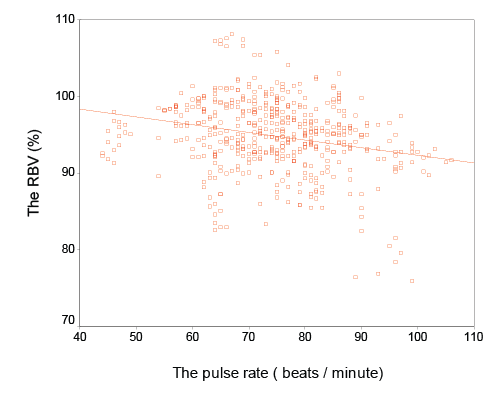
Figure 1: Correlation between the Relative Blood volume and the pulse rate (r=-0.4, p=0.03)
The change of systolic blood pressure (ΔSBP= Predialytic SBPPostdialyticSBP) increased while the RBV decreased at the end of dialysis (r= -0.5, p=0.007) (Figure 2).
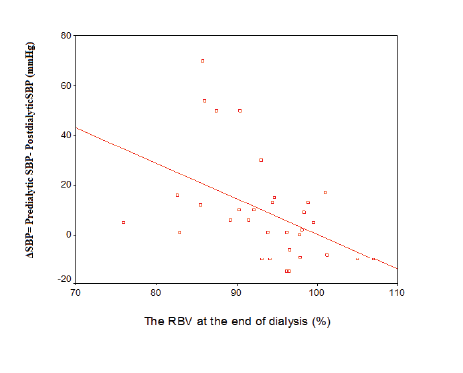
Figure 2: Correlation between the RBV at the end of dialysis and change of systolic blood pressure (ΔSBP= Predialytic SBP- Postdialytic SBP) (r=-0.5, p=0.007)
Intradialytic hypotension was more frequent with low RBV (the minimal RBV was 86 ± 2% in case of hypotension versus 95 ± 6% without hypotension p=0.04).
Like other studies we found that there was a significant correlation between RBV with heart rate and ΔSBP, and intradialytic hypotension (IDH) was more frequent with low RBV [7-10]. This suggests that a reduction in RBV through ultrafiltration stimulates cardiovascular defense mechanisms such as peripheral vasoconstriction and increasing heart rate and cardiac output. But when these mechanisms are exceeded we assist to hemodynamic instability with the RBV reduction below a critical threshold [10,11]. The strong direct correlation between plasma refilling rate (PRR) and ultrafiltration volume (UF) clearly indicates that PRR is UF dependent, and an imbalance between UF and PRR is usually the initiating factor for IDH [12]. Thereby the control of RBV can improve intra-treatment hemodynamic stability during dialysis sessions [13].
In this study the composition and the rate of vascular refilling from the extravascular compartment was studied, and showed that 87% of the fluid removed by ultrafiltration had been refilled from the extra vascular space (84% of refilling was form extracellular volume and 16% from intracellular space). It also showed that changes in blood volume occurring during dialysis may be multifactorial and several patient-related parameters and technique-related variables can affect the refilling rate. The PRR was dependent on the serum protein (so the oncotic pressure). According to Starling’s hypothesis, fluid shifts between the capillaries and the interstitium are determined by the sum of colloid-osmotic and hydrostatic pressure gradients and by the filtration coefficient of the capillary wall. Therefore, a volume larger than that withdrawn by ultrafiltration has to be refilled in order to attain colloid-osmotic equilibrium [14]. The filtration coefficient of the capillary wall can be influenced by individual nervous system, the structure of the veins or change in body temperature [4], and may explain the impact of diabetes and elderly on RRR in our study. This is supported by Winkler RE which has shown that BVM can improve clinical parameters for adequacy of haemodialysis in diabetic patients [15].
Modifications in blood flow distribution or tissue hydration strongly affect changes in plasma volume [16]. In our study the PRR was also dependant on extracellular hydration status, it was correlate to interdialytic weight gain, extracellular water, total body water before dialysis and UF rate. The results reported in this study confirm previous work by Lopot et al. [17] and Steuer et al. [18] in showing that patients who do not exhibit a substantial reduction in blood volume during hemodialysis remain over hydrated at the end of dialysis. De Vries et al. [19] have shown that intradialytic changes in blood volume were larger in dehydrated and normohydrated patients than in over hydrated patients [19]. The primary hypothesis of this study was that intradialytic changes in blood volume and postdialytic refill of the vascular compartment are both indirectly controlled by the extracellular fluid status and may indirectly reflect the hydration status of the extracellular fluid compartment, and therefore dry weight assessment [20].
In our study the change of sodium concentration influences the refilling rate. Indeed it is known that sodium is a potent osmotic regulator of water distribution within the body, and transcellular fluid shifts in HD patients [21]. Increasing of dialysate sodium concentration was proposed to remove water from intracellular space with aim to optimize the refilling and hemodynamic stability [6,22].
In this study the composition and the rate of vascular refilling in dialysis was studied, and showed that several patient-related parameters and technique-related variables affect the PRR, and their control can improve intra-treatment hemodynamic stability during dialysis sessions.
No conflict of interest.
Download Provisional PDF Here
Article Type: Research Article
Citation: Alayoud A, Maoujoud O, Aattif T, El Kabbaj D, Benyahia M, et al. (2016) Plasma Refilling in Hemodialysis: Determinant Factors and Effects on Hemodynamic Stability. Int J Nephrol Kidney Failure 2(3): doi http://dx.doi.org/10.16966/2380- 5498.132
Copyright: © 2016 Alayoud A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Publication history:
All Sci Forschen Journals are Open Access