Abstract
Background: Patients with sleep disorders report worse self-perceived quality of life than those without the disorders. In addition, it has
recently been shown that poor quality of sleep is a predictive factor of near-term mortality in patients with end stage renal disease (ESRD).
The aim of this study was to evaluate possible relationships between sleep disorder, quality of life and chronic pain in elderly diabetic
hemodialysis patients.
Methods: In the present study, the following groups were enrolled: 36 non-haemodialysis patients (group Non-HD) and 38 hemodialysis
patients (group HD). The Pittsburgh Sleep Quality Index (PSQ-I) for sleep disturbances and health-related quality of life (HRQoL) questionnaires
were administered in all the patients and their tolerability of pain was objectively evaluatedwith an algometer.
Results: In the HD group, a statistically significant positive correlation was observed between SF-36 and the maximum pain level (r: 0.723;
p<0.05). Similarly, a statistically significant negative correlation was reported between the PSQI and maximum pain level (r: -0.795; p<0.05).
Conclusion: These data suggest the existence of a close relationship between pain tolerance with sleep disorders and health-related quality
of life, in elderly diabetic patients with ESRD. Thus, the appropriate management of chronic pain in these patients could significantly improve the
quality of life and ameliorate the sleep disorder.
Keywords
Elderly diabetics; Sleep disorders; Quality of life; Algometer
Introduction
Sleep disorders are present in 65% of dialysis patients [1]. These include
delayed sleep onset, frequent awakening, restlessness and daytime sleepiness
[2]. Patients with sleep disorders report worse self-perceived health-related
quality of life than those without the disorders [1]. In addition, it has recently
been shown that poor quality of sleep is a strong predictor of near-term
mortality in patients with end stage renal disease (ESRD) [3].
Chronic pain represents a common health challenge experienced by
patients with chronic kidney disease (CKD) and seems to be associated
with poor quality of sleep [4,5]. Impaired pain threshold and sleeplessness
are both frequent symptoms in patients CKD [6,7]. Although there
are several studies in dialysis populations on the pain perception, its
relationship with quality of life and quality of sleep is still unclear. In
addition, objective pain evaluation with the Algometer was not previously
evaluated in CKD patients.
Thus, the present study seeks to evaluate a possible relationship between
threshold objective pain with sleep disorders and health-related quality of
life, in both non-haemodialysis and haemodialysis patients.
Methods
Patient population
In the study protocol, a total of 74 elderly type 2 diabetic CKD (stage
G3b-4, G5) [8] subjects were enrolled in the study. All patients were
clinically assessed by medical history, physical examination, and routine
laboratory test and by comprehensive geriatric assessment. Diabetes was
diagnosed in the last 20 years. Eligibility criteria of all patients include:
age >65 years; no evidence of chronic pain, as evaluated by McGill
questionnaire (McGill score =2), no evidence of depressive symptoms
(GDS-15<5), no evidence of malnutrition, as evaluated by albumin levels
>3.5 g/dL; maintaining functional independence or loss of it in only one
of the six basic activities of daily living (ADL); no evidence of significant
cognitive impairment, as evaluated by Mini Mental State Examination
(MMSE) >24; no evidence of severe diseases that might highly influence
mood state (e.g.: cancer, symptomatic cerebro-vascular disease with
residual deficit, schizophrenia and other psychoses); disease severity, as
evaluated by Cumulative Illness Rating Scale for overall illness severity
(CIRS) <3 (moderate). Since haemodialysis patients are characterized by
an elevated comorbidity index (CIRS-CI) that has an impact on depressive
symptoms, we compared them with elderly patients with a similar level
of CIRS-CI such as the hospitalized group. The exclusion criteria are:
oncology-related chronic pain disorder or the use of opioid analgesic,
major anti-depressive or anxiolytic agents. The purpose of the study was
explained to all subjects and their voluntary written consent was obtained
before their participation. The study protocol was reviewed and approved
by Ethical Committee of our Institution (121/2014).
Experimental protocol
For the present study, the following groups were enrolled: 36 nonhaemodialysis
patients (group Non-HD) with mean age of 75 ± 3 years,
body mass index( BMI) of 26.5 ± 3 Kg/m2
, systolic blood pressure(SBP) of
125 ± 09 mmHg, diastolic blood pressure(DBP) of 80 ± 4 mmHg, HBA1c
of 6.8 ± 0.4% and GDS-15 of 3 ± 1, and 38 hemodialysis patients (group
HD) with mean age of 76 ± 2 years, BMI of 26.1 ± 2 kg/m2
, SBP of 122 ±
08 mmHg, DBP of 81 ± 3 mmHg, HBA1c of 6.7 ± 0.5% and GDS-15 of 4 ±
2. At the baseline, all patients had no chronic pain, as evaluated by McGill
questionnaire. The clinical characteristics are reported in Table 1.
Cognitive function was evaluated by a Mini Mental State Examination
(MMSE), a widely used screening instrument for measuring global
cognitive status [9]. It is composed of 30 items divided in two sections.
The first section requires vocal responses and covers orientation, memory
and attention. The second part focuses on the ability to name, to follow
verbal and written commands, to write a sentence and a copy a complex
polygon. Maximum total score is 30 and a score less than 24 indicate a
significant cognitive impairment [9], although other rating scores have
been employed. Screening tests to assess depressive symptoms include
the Geriatric Depression Scale 15 items (GDS-15) [10]. The GDS was
designed specifically assesses depressive symptoms in elderly patients
[11]. GDS-15 represents a shortened version consisting of 15 questions
[12] that has been found to correlate highly with the original 30-item
instrument [13]. The GDS-15 is a reliable and well-validated measure of
depression in elderly [10,14] and in CKD patients [15]. Responses were
coded 1 = has symptom; vs. 0 = symptom not present. GDS-15 score >5
indicate depressive symptoms [10]. To assess physical illness we used the
Cumulative Illness Rating Scale (CIRS) [16]. This measure evaluates the
cumulative score derived from ratings of the degree of impairment in each
of 13 major organ groups (cardiac; hypertension; vascular; respiratory;
eye-ear-nose-throat-larynx; upper GI; lower GI; hepatic; renal; uretersbladder-urethra-prostate-genitals;
musculo-skeletal-integumentary;
neurological; endocrine-metabolic) and impairment in the psychiatricbehavioral
category [17]. In particular, the CIRS evaluates the clinical
rating of impairment as follows: no impairment = 1; mild impairment =
2; moderate impairment = 3; severe impairment = 4 and extremely severe
impairment = 5. Two CIRS summary measures were evaluated, each
excluding the psychiatric/behavioural item to avoid confounding with
mental health and cognitive functioning. First, overall illness severity is
represented by the mean of the 13 CIRS items. Second, the comorbidity
index (CIRS-CI), which reflects the diversity of severe illnesses, is the
total number of the 13 categories in which moderate, severe or extremely
severe impairment levels of disease are recorded. The functional status
is evaluated by Activities of Daily Living (ADL) [18]. It consists of six
items for evaluation of self-care, which measure the functional status
as dependence in each of the six ADL. Dependence is defined as no
ability to manage the following activities: bathing, toileting, transferring,
continence, dressing and eating. One point was assigned for each of
activities in which a subject was dependent, ranging from 0 (independent
in all activities) to 6 (totally dependent).
The Pittsburgh Sleep Quality Index (PSQ-I) [19] screens for sleep
disturbances over a 1-month period. There are 19 questions with seven
component scores including ‘subjective sleep quality, sleep latency, sleep
duration, habitual sleep efficiency, and sleep disturbance, use of sleeping
medication and daytime dysfunction’. The seven component scores are
summed to yield one global score [19]. A global score >5 has 89.6%
sensitivity and 86.5% specificity for determining disturbance in sleep [19].
The PSQI has previously been used in CKD patients [20].
Participants were asked to complete a HRQoL questionnaire (SF-36v2
Health Sur- vey) [21]. The SF-36 has been used extensively in patients with
kidney disease and has sound psychometric characteristics in this patient
population to assess HRQoL [22]. The SF- 36 is a well-validated 36-item
questionnaire covering issues relating to physical, psycho- logical and
social functioning that generates scores from 0 (worst) to 100 (best) for
eight sub-scales of HRQoL: physical functioning (PF), role-physical (RP),
bodily pain (BP), general health (GH), vitality (VT), social functioning
(SF), role-emotional (RE) and mental health (MH). It is composed of
two component summary scores, the Physical Component Summary
(PCS) and Mental Component Summary (MCS). Higher scores indicate
better HRQoL [23]. The McGill questionnaire was designed to provide
quantitative measures of clinical pain. It consists primarily of major 3
classes of word descriptors-sensory, affective and evaluative- that are
used by patients to specify subjective pain experience. It also contains
an intensity scale and other items to determine the properties of pain
experience. In addition the questionnaire comprised a top sheet to record
necessary medical information (such as diagnosis and drug intake), line
drawings of the body to indicate the spatial distribution of the pain, words
that describe temporal properties of pain, and the overall present pain
intensity (PPI). The PPI is recorded as a number from 1 to 5, in which each
number is associated with the following words: 1, mild; 2, discomforting;
3, distressing; 4, horrible; 5, excruciating. This questionnaire initially
requires 15-20 min, with increasing experience; it is completed in 5-10
min [24]. The pain threshold is evaluated with algometer [25]. This
instrument consisted of a 0.8 mm needle with a round tip, fixed in a
vertical position to a force transducer (FSG, Honeywell, Columbus,
OH, USA). The signal from the sensor was amplified by an instrumental
amplifier (INA 125, Burr Brown, Dallas TX, USA and digited with an A/D
converter (MAX 147, Maxim, Sunnyvale, CA, USA). This apparatus was
encased in a small plastic box (10x15x3 cm) and connected to the parallel
port of a personal computer. Custom software, written with LabView
(National Instruments, Austin, TX USA), permitted the display, in real
time, of the force applied to the transducer over time and the recording
of the data for subsequent analysis. The system was calibrated before each
use with two known loads (0 g and 220 g). The testing procedure required
the subject to auto-administer increasing force on the testing needle
until a pain sensation was realized (minimal test). This test was repeated
eight times, respectively, on the tip and on the dorsal surface of the third
phalanx of the second, third, fourth and fifth finger. These eight tests were
then repeated, but the subjects were asked to push on the needle until the
maximum tolerable sense of pain was reached (maximal test). For each
test, the maximal applied force (in grams) was defined as “pain threshold”
and used for statistical analysis. Thus, two pain thresholds were evaluated:
one for the minimum pain level and the other for the maximum pain
level. To evaluate the overall tolerability of the described protocol, and
the end of every procedure, each subjects was asked to quantify on a 0 (no
discomfort) to 10 (intolerable pain) scale, the overall discomfort as a result
of the exam. All questionnaires and pain threshold are performed in the
interdialytic day.
Statistical analysis
All values were expressed as the mean ± standard error. Comparison
between groups was performed using unpaired Student’s “t” test. ANOVA
was performed to evaluate the homogeneity of sample. Difference at the p
< 0.05 level of probability were considered significant.
Results
Body Mass Index (BMI), Systolic Blood Pressure (SBP), Diastolic
Blood Pressure (DBP), HBA1c, serum creatinine, Blood Urea Nitrogen
(BUN), Geriatrics Depression Scale (GDS-15), McGill Subjective Pain
Questionnaires and SF-36 (SF-36v2 Health Survey), were all similar in
both non haemodialysis (Non-HD) and in haemodialysis (HD) groups
(p: ns), and the mean values are reported in Table 1.
Minimum pain level (an index of pain sensitivity) was of 456 ± 23 gin
the Non-HD group and it was significantly increased in the HD group to
615 ± 27 g(p<0.05).
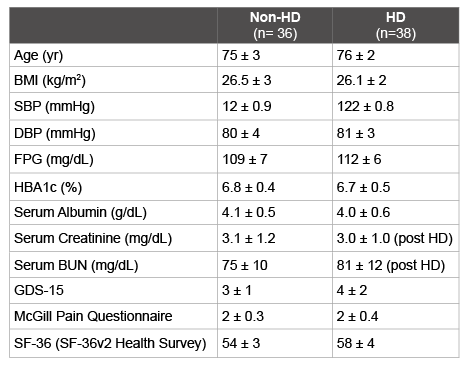
Table 1: Clinical characteristics of Non-Haemodialysis (Non-HD) and
Haemodialysis (HD) groups.
Body mass index (BMI), Systolic Blood Pressure (SBP), Diastolic Blood
Pressure (DBP), Fasting Plasma Glucose (FPG), Blood Urea Nitrogen
(BUN), Geriatrics Depression Scale (GDS-15). Values are mean ± SEM.
Similarly, maximum pain level (an index of pain tolerance) was of 542
± 24 g in theNon-HD group and it was significantly increased to 749 ± 31
g in the HD group (p<0.05).
PSQI (an index of sleep disorders) was 5 ± 1 in the Non-HD group and
it was significantly increased to 23 ± 2 in the HD group (p<0.01).
In the HD group, a statistically significant positive correlation was
observed between SF-36 and the maximum pain level(r: 0.723; p<0.05).
In addition, a statistically significant negative correlation was reported
between the PSQI and maximum pain level(r: -0.795; p<0.05) (Figure 1).
Discussion
In this study we observed in the HD group, a significant positive
relationship between the quality of life (as evaluated by SF-36) and
maximum pain level (an index of the pain tolerance). This observation
highlights the crucial role of pain level in the quality of life in these
patients. A recent study has in fact suggested that patients’ perception of
pain, may be more important than objective assessment in determining
the quality of life of patients with ESRD [26]. Chronic pain represents a
common disorder in CKD patients [27], especially in patients with endstage
renal disease (ESRD) [28]. In this regard, it has been reported that
about 80% of ESRD patients undergoing dialysis had chronic pain [29]
and 35-70% of patients had moderate to severe chronic pain [30]. There
is evidence that symptoms (such as pain) are both under recognised [29]
and under treated [31] in the dialysis population. Although there are
several studies on sleep complaints and disorders and their relationship
to pain level in chronic kidney disease (CKD) patients, the objective
perception of pain, as evaluated by the algometer has not been studied
in dialysis populations before. In our population, we did not observe
significant differences in the subjective chronic pain, as reported by the
use of McGill questionnaires. These data strongly suggest that subjective
pain evaluation could under recognise the real pain in these subjects. Pain
is a subjective feeling that is therefore particularly difficult to quantify and
study [32]. For instance,different studies have reported that pain level, as
evaluated by BPI questionnaire pain and McGill Pain Questionnaire (SF
MPQ), could be under-recognized in these patients [29,33]. No studies
have focused on the specific problem of pain in the dialysis population,
using the algometer its evaluation. The algometer is quick and easy to
execute and does not require complicated training for the examiner and
is virtually free of any mechanical interference from the tester [25]. In
the present study, we report the pain level, evaluated by an algometer,
in elderly haemodialysis patients; demonstrating that an increased pain
threshold characterizes ESRD patients.
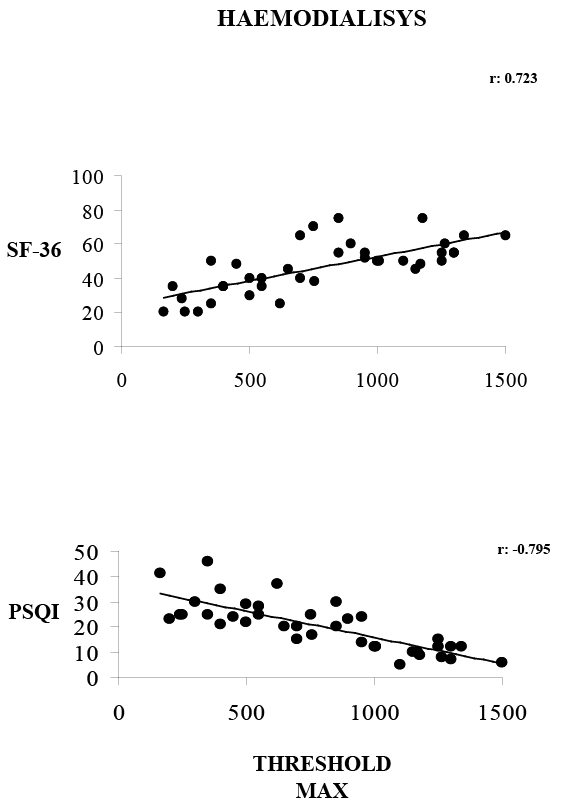
Figure 1: A statistically significant positive correlation between
threshold pain maximum and SF-36 (r: 0.723). A statistically
significant negative correlation between PSQI and threshold pain
maximum (p<0.005; r: -0.795).
Persistent pain may lead to sleep disorders. Sleep complaints and sleeprelated
disorders are common in dialysis patients [34]. In previous studies
it has been observed that a substantial proportion of ESRD patients
reported pain during off dialysis (44%) time and poor sleep (45% have
a PSQI score of >5) [35,36]. Chronic pain and sleep disturbances each
independently have profound detrimental effects on quality of life (QoL)
in ESRD populations [37]. In particular, we observed, in HD patients, an
increased maximal pain threshold (an index of increased tolerability) that
is associated with a less sleep disorder, as evaluated by PSQI.
In conclusion the present study has demonstrated the utility of an
algometer in the objective evaluation of pain tolerance, which may be
underestimated by subjective methods of evaluation. The study has also
shown that pain tolerance is directly related to quality of life (SF-36) and
inversely related to sleep disorder (PSQI).
These data suggest the existence of a close relationship between chronic
pain/pain tolerance with sleep disorders and health-related quality of life
in ESRD patients. Thus, appropriate management of chronic pain in these
patients could significantly improve their quality of life and ameliorate
their sleep disorders.
Article Information
Article Type: Research Article
Citation: Giordano M, Ciarambino T, Pace MC,
Passavanti MB, Viggiano A, et al. (2015) Relationship
between Pain Tolerance/ Sleep Disorders and Quality
of Life in Elderly Diabetic Haemodialysis Patients.
Int J Nephrol Kidney Failure 1(3): http://dx.doi.
org/10.16966/2380-5498.114
Copyright: © 2015 Giordano M, et al. This is an
open-access article distributed under the terms
of the Creative Commons Attribution License,
which permits unrestricted use, distribution, and
reproduction in any medium, provided the original
author and source are credited.
Publication history:
Received date: 11 June 2015
Accepted date: 11
Sep 2015
Published date: 15 Sep 2015



