Figure 1: The image was republished 25 years later; commented in the text. Online: HYPERLINK “http://www.moluch.ru/archive/63/9770/” ”http://www.moluch.ru/archive/63/9770/”

Sergei V Jargin*
Peoples’ Friendship University of Russia, Moscow, Russia*Corresponding author: Jargin SV, Peoples’ Friendship University of Russia, Clementovski per 6-82; 115184 Moscow, Russia, E-mail: sjargin@mail.ru
Insufficient international coordination of medical research can result in repetition of studies performed in other countries. Renal biopsy is a valuable diagnostic method; it was broadly used for research in the former Soviet Union. The number of biopsies has decreased since 1990; but medical research is on the increase today. Therefore, the purpose of this review was to remind that, performing renal biopsy or other invasive procedures, the risk-to-benefit ratio should be kept as low as possible. Renal biopsies were taken for research from patients with glomerulonephritis, pyelonephritis, amyloidosis, renovascular hypertension (in some studies from both kidneys), essential hypertension, alcoholism, diabetes mellitus, in congenital hydronephrosis and other urinary tract anomalies. About one third of the biopsy cylinder was routinely embedded in epoxy resin. The epoxy resin sections were made for research but not used for diagnostics, the latter being performed mainly on the basis of paraffin sections and immunofluorescence. Extensive use of renal biopsies without silver impregnation and electron microscopy was accompanied by over diagnosis of glomerulonephritis and corresponding overtreatment. Furthermore, the concept of hypoplastic renal dysplasia, developed on the basis of renal biopsy, probably interfered with the morphological diagnosis of Alport syndrome.
Renal biopsy; Pyelonephritis; Alport syndrome; Hydronephrosis; Alcoholism
Insufficient international coordination of medical research and partial isolation from the international scientific community can result in repetition of studies performed in other countries. Renal biopsy (RB) is a valuable diagnostic tool; it was broadly used in the former Soviet Union (SU). Today there are more funds for research; and medical science is on the increase. Under these circumstances, the purpose of this review was to recollect some experience from the recent past to ensure more responsible attitude in future and to remind that, performing RB or other invasive procedures, the risk-to-benefit ratio must be kept as low as possible.
RB were taken for research from patients with glomerulonephritis (Gn), pyelonephritis, amyloidosis, renovascular hypertension from both kidneys in some studies [1-6], essential hypertension [2], in certain studies with mild proteinuria and/or hematuria [7,8], alcoholism [9-17], diabetes mellitus [18], rheumatoid arthritis [19], and from children with urinary tract anomalies including those combined with hydronephrosis or pyelonephritis [20-23]. RB for research from both kidneys in renovascular hypertension was commented on previously [24,25].
As discussed below, electron microscopy was infrequently used for diagnostics in the former SU. Nevertheless, about one third of the biopsy cylinder was embedded in epoxy resin. The semi-thin epoxy resin sections were made for research but were not used for diagnostics, the latter being performed mainly on the basis of paraffin sections and immunofluorescence.
In the studies [26,27], excisional RB were sampled during kidneypreserving operations such as lithotomy from patients with chronic or acute (including purulent) pyelonephritis. In the literature, pyelonephritis is not listed among conditions where RB is indicated, while acute inflammation, infection and hydronephrosis are generally considered to be contraindications [28-30]. In the study [31], RB was taken from patients with chronic pyelonephritis and hydronephrosis, while conclusions were based on linear correlations between ultrastructural morphometric and clinical indices. However, statistical significance of the correlation coefficients in this and some similar studies was overstated. A comparison with the reference tables [32] demonstrated that many claimed P-values were exceedingly high for the given values of the correlation coefficient and the number of correlation pairs [31,33-35]; more details are in [25]. In a more recent study, “cytomembranes of the interstitial tissue of renal medullary layer” were studied in core RB collected during lithotomies from patients with urolithiasis and secondary pyelonephritis [36]. Core RB in pyelonephritis were taken also by other researchers [37]. Fineneedle RB in acute pyelonephritis was performed and recommended in the recent study [38].
Among persons with alcoholism, biopsies were taken from kidneys, pancreas, liver, lung, salivary glands, stomach and skin, repeatedly in some cases [12,13,17]. It was concluded on the basis of a series of RB studies that a generalized cytoskeleton abnormality with accumulation of intermediary filaments in macrophages, epithelial and other cells is typical for the cell damage by ethanol or the “alcoholic disease” [9,12,13]. It is known that Mallory bodies, seen in alcoholic hepatitis and some other liver conditions, are composed of intermediate filaments; however, generalizations [9,12,13] have never been confirmed. In any case, a cytoskeleton could have been studied in experiments or post mortem. Another example: RB were collected from 40 patients with chronic alcoholism and nephritic symptoms, whereas “intracapillary proliferative glomerulonephritis” was diagnosed in all cases [15]. In another study by the same researchers, the histopathological findings in 40 from 43 patients with alcoholism and nephritic symptoms were morphologically classified as membranoproliferative (mesangiocapillary) Gn; while in 29 from 31 patients with nephritic symptoms without alcoholism “fibroplastic” Gn was diagnosed [16]. The striking difference between the two groups appears to be unusual. Other invasive procedures (celiacography, endoscopic cholangiopancreatography etc.) were applied in alcoholic patients without clear indications [17]. In the author’s opinion, repeated biopsies from different organs, doubtful morphological descriptions and interpretations, give reasons to question the indications for RB at least in a part of the studied patients with alcoholism.
RB were taken without sufficient indications also in some cases of suspected Gn, which is less obvious because in the Russian-language literature RB has been generally regarded to be indicated in suspected Gn [39-41]. In the internationally used handbooks, however, RB in isolated proteinuria and/or microhematuria without abnormal urine sediment or signs of progressive renal disease is generally regarded as not indicated [28,42,43]. Indications for RB are sometimes formulated more liberally [29]; but an obvious precondition must be a high quality of morphological examination.
In the former SU, RB were sometimes collected from patients with “inactive nephritic” or latent clinical forms of Gn with proteinuria and/ or hematuria [39,44-47]. At the same time, the classification of Gn has been different from that used internationally [30,43,48], which obviously interfered with implementation of practical recommendations from the international literature. For example, Gn classification applied in the former SU did not consider IgA nephropathy as a separate entity [39,49-51]. IgA-nephropathy was not mentioned even in the article from a leading institution dedicated to the “hematuric form” of Gn [52]. IgA nephropathy was usually diagnosed on RB as mesangioproliferative Gn (MG) and sometimes treated with corticosteroids and/or cytotoxic drugs [39,53-60]. In newer handbooks controversies can be found; for example, in the textbook [61], IgA nephropathy and Berger disease are discussed separately and different treatments are recommended. In the textbook [41], it is written in one place in regard to the therapy of MG: “Influence of immunosuppressive drugs has not been proven”, and in another place of the same chapter: “Efficiency of cytotoxic drugs has been proven” [41]. In the “National Manual” [62], probably the most authoritative Russianlanguage edition in nephrology, IgA-nephropathy and MG are discussed in one chapter titled “Mesangioproliferative (IgA) glomerulonephritis” (from Russian): “The term IgA nephropathy is used to designate an entity, the morphological equivalent of which is mesangioproliferative glomerulonephritis” [62]. It is partly at variance with the international literature, according to which glomeruli in IgA nephropathy may be normal at light microscopy or may show segmental mesangial proliferation confined to some glomeruli (focal proliferative Gn), diffuse mesangial proliferation (MG) or, rarely, crescentic Gn. Heeling of focal lesions may produce a picture of focal sclerosis [43,63,64].
The diagnosis of MG was used broadly, encompassing 49-60.8% of all Gn cases diagnosed by RB [50,65,66]. Epoxy resin sections and silver impregnation were not used for the diagnostics, while electron microscopy was applied only occasionally. By means of these methods, the pool of MG could have been partly sorted out, excluding from it the cases morphologically bordering on the norm i.e. isolated proteinuria and/or hematuria without renal or systemic disease, not requiring immunosuppressive therapy. In such cases, histologically are often detected only minor glomerular abnormalities: mild mesangial widening and hypercellularity, scarce deposits of immunoglobulins and the complement [67]. In conditions of insufficient quality of histological specimens, without silver impregnation and electron microscopy, such changes and, correspondingly, Gn, were sometimes overdiagnosed. Data reported in the study, where percentages of glomerular diseases diagnosed by RB were compared between Moscow and Rostock in Germany (Table 1), are suggestive of over diagnosis of Gn and MG in particular. Outdated equipment, such as sledge microtomes from the 1930s, was used in many institutions. The paraffin slides were relatively thick (around 6-7 µm), the thickness being uneven. Suboptimal standardization of the hematoxylineosin, van Gieson and PAS stains used for the diagnostics, occasional over staining, etc. can mimic a glomerular capillary wall thickening. This is apparently a reason why membranous Gn was diagnosed in Moscow more than twice as frequently as in Rostock (Table 1). The author of this review participated in research [24] using epoxy resin sections cut by an LKB pyramitome with glass knives; after that he found it difficult to evaluate diagnostic paraffin sections, less clearly visualizing basement membranes and mesangial matrix.
RB were sometimes taken from patients with the “inactive nephritic” or latent clinical types of Gn i.e. with minimal proteinuria and/or hematuria [39,44-47]. In some studies, patients with the inactive or latent clinical types of Gn, isolated proteinuria or hematuria, were treated and recommended to be treated by corticosteroids and/or cytotoxic drugs such as azathioprin, cyclophosphamide or chlorambucil [53-60], which sometimes amounted to overtreatment.
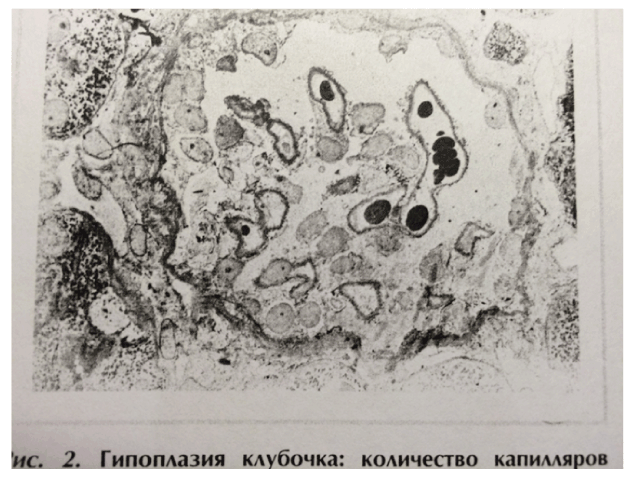
The dubious concept of hypoplastic renal dysplasia was developed on the basis of predominantly pediatric RB. It was described as follows: “Racemosely arranged glomeruli with single capillary loops, abundant rounded cells freely lying in the cavity of a capsule; single mesangial cells; irregular enlargement, loosening, and thinning of the basement membrane”, narrow extracapillary space [68], glomeruli having irregular form and singular capillary loops [69] or total absence of capillaries [68], which has no analogues in the international literature, where the terms “renal hypoplasia” and “dysplasia” are used with a different meaning [70- 73]. In the author’s opinion, the descriptions were at least in part based on tangential sections of glomeruli, which is evident looking at the illustrations [68,69], reproduced [74]: http://www.moluch.ru/archive/63/9770/ (accessed 7/28/2015). Interestingly, the same ultrastructural image (Figure 1) [68] was reproduced with a similar legend by other authors in the same journal 25 years later [75] (compare Figure 1 in [74] available online).
The common feature of these and some other works is presentation of ultrastructural findings without confrontation with light-optical equivalents; whereas variants of the norm and artifacts are sometimes interpreted as characteristic pathological phenomena. For example, hypoplastic dysplasia was diagnosed by electron microscopy in 8 from 34 randomly selected patients aged 9-54 years with nephrotic syndrome and histologically minimal glomerular changes [76]. At the same time, there were no cases of Alport syndrome or thin basement membrane nephropathy (having some morphological feature in common with “hypoplastic dysplasia” [68,69]) among 4440 RB including 2770 cases of glomerular disease overviewed [65]. These two conditions constituted more than 1% of all renal diseases diagnosed by RB in Rostock [77]. The concept of hypoplastic dysplasia, discussed with clinicians performing biopsies, could have interfered with the diagnosis of thin basement membrane nephropathy and Alport syndrome, the latter being of importance because of genetic implications.
Condition |
Moscow |
Rostock |
Diffuse Gn |
81.7 |
59.3 |
MG |
55.5 |
40.2 |
Membranous Gn |
9.2 |
4.1 |
Minor glomerular |
7.1 |
20.8 |
Table 1: Percentages of glomerular diseases diagnosed by RB in Moscow and Rostock in the years 1978-1983

Figure 1: The image was republished 25 years later; commented in the text. Online: HYPERLINK “http://www.moluch.ru/archive/63/9770/” ”http://www.moluch.ru/archive/63/9770/”
Today, the same researchers (or their heirs) apply the term hypoplastic dysplasia to the glomerular changes in congenital hydronephrosis and other renal abnormalities in children, interpreting them as inborn nephropathy affecting a major part of glomeruli [20,22,75,78,79]. Note that a regular combination of two prima facie unrelated conditions: an inborn glomerulopathy affecting a major part of glomeruli, and hydronephrosis related to an abnormality of the ureteropelvic junction, seems to be improbable. For the latter research, 167 intra-operative wedge or core RB from children with urogenital malformations, plus 18 RB for the control group from adult patients undergoing urological operations, were collected [79].
The same research group collected 60 pancreatic excision biopsies 5 × 5 mm in size [80] during the surgical operations of “pancreatic blood shunting into the systemic blood flow in insulin-dependent diabetics” [81]. From the same patients, 51 core RB were taken [80]. Apart from several reports from the former SU, we have found in the literature no analogues of this surgical treatment modality of diabetes mellitus discussed [82]. Morphological descriptions of pancreatic and renal biopsies in type 1 diabetes mellitus included the following: islets of Langerhans “containing B-cells with destructive changes” [83], presence of endocrine-like cells in the acini and among the cells of the inter-acinar ducts [84,85], Gn and mesangiolysis as consecutive stages of diabetic glomerulosclerosis [86], frequent mesangial interposition with displacement of mesangial cells to the peripheral capillary loops and formation of double-contour glomerular basement membranes [86,87], which is at variance with usual morphological descriptions [88-92]. In particular, the morphological picture of Gn, if detected in a diabetic patient, is usually interpreted as a superimposed condition [90,91]. It should be commented that in diabetes mellitus, RB is generally indicated for patients under the suspicion of a renal disease other than diabetic nephropathy [93]. It is important to identify a non-diabetic renal condition, in particular, membranoproliferative Gn (characterized by mesangial interposition), where immunosuppressive therapy should be considered. Therefore, representing the morphological picture of Gn with mesangial interposition as a characteristic phenomenon or a stage of diabetic nephropathy can be misleading.
The RB material used in some studies discussed above was unique e.g. wedge or core biopsies in hydronephrosis, acute and chronic pyelonephritis. Apart from the articles discussed here, no other studies based on RB in hydronephrosis and acute pyelonephritis are known to us, while in chronic pyelonephritis no other studies performed abroad later than in the 1960s have been found. There is an opinion, shared by the author, that, considering potential complications, “RB for research” should not exist as such; it must always be done according to clinical indications. If a patient gives informed consent to research on renal tissue obtained for diagnostic purposes, it can be done, provided that enough tissue remains for the diagnostics, if non-morphological or otherwise suboptimal for the diagnosis research methods, consuming the tissue, are applied. It should be commented that even today, in spite of official regulations; the principle of informed consent is often disregarded. At a reception of some governmental medical institutions a patient is given a form, where he or she must beforehand give a written consent to all diagnostic and therapeutic procedures, which are then sometimes performed in spite of the patient’s objections. In conclusion, high level of integrity, quality of specimens and of their examination must be a precondition for RB research. This review has discussed several studies from the past, but the problem is still with us: invasive procedures performed without sufficient clinical indications and informed consent [82].
Download Provisional PDF Here
Aritcle Type: Review Article
Citation: Jargin SV (2015) Some Aspects of Renal Biopsy for Research. Int J Nephrol Kidney Failure 1(2): doi http://dx.doi.org/10.16966/2380-5498.108
Copyright:© 2015 Jargin SV. This is an openaccess article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Publication history:
All Sci Forschen Journals are Open Access