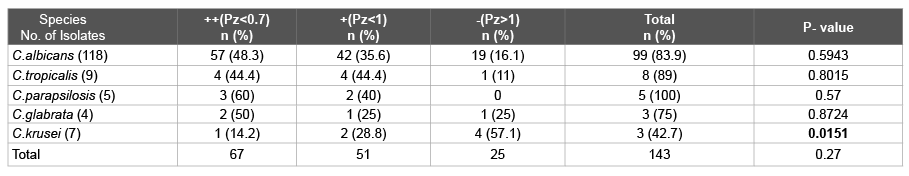
Table 1: Phospholipase activity exhibited by Candida isolates
Phospholipase activity Pz<1.00 (Positive), Pz<0.7 (More virulent)

Ravinder Kaur* Ritu Goyal Megh S Dhakad Preena Bhalla Richa Diwan
Department of Microbiology, Maulana Azad Medical College & Associated Lok Nayak Hospitals, New Delhi, India*Corresponding author: Dr. Ravinder Kaur, MBBS, M.D, Director Professor, Department of Microbiology, Maulana Azad Medical College & Associated Lok Nayak Hospitals, New Delhi,India, Tel: 011-23235751(171); E-mail: rkaur.mamc@gmail.com
Objectives: Candida species, considered as one of the most pathogenic yeasts, is frequently isolated from the immunocompromised patients. The aim of the study was to determine proteinase, phospholipase and adherence activities by Candida species isolated from HIV/AIDS patients that contribute both to the pathogenesis of superficial and systemic candidiasis.
Material and methods: The present investigation deals with a detailed study of three hundred and fourteen samples recovered from diverse clinical sources viz; sputum, blood, urine, oral swab and CSF samples from HIV/AIDS patients studied. The identification of Candida species was done by germ tube test, cornmeal agar test, sugar assimilation and fermentation tests and Vitek-2 yeast identification system. Proteinase and phospholipase estimation and adherence assay was determined for all the isolates.
Results: One hundred and forty three isolates of Candida species were recovered from three hundred and fourteen diverse clinical sources. C.albicans (82.51%) was the predominant species followed by C.tropicalis (6.29%), C.krusei (4.89%), C.parapsilosis (3.49%) and C.glabrata (2.79%). 100% C.parapsilosis >89% C.tropicalis >83.9% C.albicans >75% C.glabrata >42.8% C.krusei exhibited phospholipase activity whereas 80% C.parapsilosis >77% C.tropicalis >75% C.glabrata >61.1% C.albicans >42.8% C.krusei exhibited proteinase activity and 83.8% C.albicans >80% C.parapsilosis >77.7% C.tropicalis >75% C.glabrata >71.4% C.krusei exhibited adherence activity.
Conclusion: The present study showed an isolation of C.albicans followed by C.tropicalis, C.krusei, C. parapsilosis and C.glabrata in different clinical samples from HIV/AIDS patients. The overall numbers of C.albicans producing proteinase, phospholipase and inducing biofilm formation were more than the number of Candida Non. albicans producing these virulence factors. This result suggests that the biofilm production is important for Candida species in addition to other mechanisms to establish infections.
Biofilm; Candidiasis; HIV/AIDS; Phospholipase; Proteinase
Instances of fungal infections have increased dramatically in the past two decades due to the acquired immune deficiency syndrome (AIDS) epidemic, improved life-sustaining technologies, and anti-cancer therapies. Unlike other pathogenic fungi with environmental niches, Candida species are found as commensal organisms on the mucosa of gastrointestinal and urogenital tracts of 60-80% of humans [1]. The most common infections caused by Candida species comprise mucosal infections followed by invasive infections. Risk factors associated with candidemia include neutropenia, cancer chemotherapy, antimicrobial agents, and prolonged indwelling catheterization [2]. Oropharyngeal candidiasis (OPC) occurs in several patient populations and it is seen with increasing frequency with decease of CD4+ cell counts usually to a threshold less than 200 cells/mm3 [3].
The morphological changes and the ability to form hyphae play a large role in the formation of biofilms. Candida species have developed an effective battery of putative virulence factors and specific strategies to assist in colonization, invasion, and pathogenesis. The virulence factors expressed by Candida species, which mainly emphasize biofilm formation, production of proteinase, phospholipase, etc. may vary depending on the type of infection, the site and stage of infection, and the nature of the host response. Once the contact is made, enzymes facilitate adherence by damaging or degrading cell membranes and extracellular proteins thus permitting the yeast to enter the host [4]. The production of biofilm is also associated with a high level of antimicrobial resistance of the associated organisms making the eradication of organisms difficult [5].
The main objectives of the study was to determine proteinase, phospholipase and adherence activities by Candida species isolated from HIV/AIDS patients that contribute both to the pathogenesis of superficial and systemic candidiasis.
Two hundred, symptomatic, confirmed human immunodeficiency virus (HIV)-positive adult patients, of both sexes, suspected of having a Candida infection were taken as a study population from Maulana Azad Medical College, New Delhi. Relevant clinical samples depending on the organ system involved like Oropharyngeal swab, sputum, blood, urine, CSF, stool were collected and subjected to direct microscopy, fungal culture and serology. Identification & speciation of yeast isolates was done by the biochemical methods.
Prospective observational study.
Ethics approval was obtained from institutional ethics committee, Maulana Azad Medical College & Associated Hospitals (Lok Nayak, GB Pant Hospital, Guru Nanak Eye Centre, & Chacha Nehru Bal Chikitsalaya) New Delhi-110002, India.
The immune status was assessed by performing the CD4 count of each patient enrolled in the study, by flow cytometry using the fluorescene activated cell sorter BD FACS Count system (Becton Dickinson) as per the manufacturer’s instructions.
The identification of Candida species isolates was done by germ tube test, Cornmeal agar test, sugar assimilation and fermentation tests using yeast nitrogen base agar (Difco, Becton Dickinson, India) as per standard recommended procedures [6-8] and by Vitek-2 yeast identification system (bioMerieux, Marcy Etoile, France).
Virulence activities comprising Proteinase assay, phospholipase estimation and adherence assay determined for all the isolates as:
Proteinase assay: Candida proteinase was detected by the slightly modified Staib method using bovine serum albumin medium (dextrose 2%, KH2 PO4 0.1%, MgSO4 0.05%, agar 2% mixed after cooling to 50°C with 1% bovine serum albumin solution). The proteinase activity was scored as grade – when no visible halo was present, grade + when visible protolysis was limited to 1-2 mm around the colony and grade ++ when the zone of proteolysis was more than 2 mm from the margin of the colony [9].
Phospholipase estimation: The isolates were screened for their extracellular phospholipase activity by growing them on egg-yolk agar and measuring the size of the zone of precipitation by the slightly modified Samaranayake et al. method [10]. Phospholipase activity (Pz value) was determined by the ratio of the diameter of the colony to the total diameter of the zone of precipitation. This ratio is designated as Pz (Phospholipase zone). If Pz is less than 1, phospholipase is produced. Pz less than 0.7 implies that the strain is more virulent [10].
Adherence assay: Adherence activity of the isolates was determined by spectrophotometeric method. Spectrophotometer reading was performed at 405nm with a microtiter plate method. The Adherence activity of each isolate was scored as either negative (% Tbloc; <5), 1+ (% Tbloc; 5 to 20), 2+(% Tbloc; 20 to 35), 3+ (% Tbloc; 35 to 50), or 4+ (% Tbloc; >=50) [4].
Statistical analysis: Statistical analysis was performed by SPSS software (version 21.0; SPSS S.L., Madrid, Spain). Data were checked for normality before statistical analysis using Shaipro Wilk test. Categorical variables were analysed using the chi square test or Fisher’s exact test as appropriate. For all statistical tests, p<0.05 was considered to indicate a significant difference. All tests of statistical significance were two tailed.
Two hundred HIV/AIDS symptomatic patients were studied. Five of our patients were Intersexes (2.5%). 111 (56.9%) males and 53 (27.1%) females were married while 25 (12.8%) males and 6 (3%) females were unmarried. One fifty five (79%) patients belonged to the age group 21-40 years, with the maximum number belonging to 26–40 years. The M: F ratio in our study was 2.3:1.
The CD4 counts ranged from 16-1033 cells/µl. 93 (46.5%) patients had CD4 counts <200 cells/µl, while CD4 count <100 cells/µl was seen in 40 (20%) and CD4 count <50 cells/µl in 20 (10%) patients depicting a major population with severe immunosuppression.
The most common presenting complaints in our study population were seen to be white oral patch (82%), weight loss (79%), fever (67%) and loss of appetite were seen in 53% patients. 99.5% patients had more than one complaints.
One hundred and forty three Candida isolates were identified with C.albicans (82.51%) being the most common species followed by C. tropicalis (6.29%), C.krusei (4.89%), C.parapsilosis (3.49%), C.glabrata (2.79%).
Phospholipase activity was detected in 118 (82.5%) Candida isolates. 83.8% C.albicans exibited phospholipase activity in comparison to 76% Candida nonalbicans species. 100% C.parapsilosis >89% C.tropicalis >83.9% C.albicans >75% C.glabrata >42.8% C.krusei exhibited phospholipase activity. The highest ++ phospholipase activity was exhibited in 60% C.parapsilosis followed by 50% C.glabrata, 48.3% C.albicans and 44.4% C.tropicalis compared to 14.2% of C.krusei are shown in Table 1.
Proteinase activity was detected in 99 (69.23%) Candida isolates. 69.4% C.albicans produced proteinase in comparison to 68% Candida nonalbicans. 80% C.parapsilosis >77% C.tropicalis >75% C.glabrata >61.1% C.albicans >57.4% C.krusei exhibited proteinase activity. Highest proteinase avtivity (++) was seen in 55.5% C.tropicalis, 29.6% C.albicans, 25% C.glabrata and14.2% C.krusei whereas (+) activity was seen in 80% C.parapsilosis results are presented in Table 2.
The plate assay showed adherence activity in 82.6% Candida isolates from patients. 1+ Adherence activity was shown by 9.1% isolates while 28.7%, 14.7%, 30.1% isolates elicited 2+, 3+, 4+ activity respectively. 4+ Adherence activities were seen in C.albicans (32%), C.krusei (28.5%), C.glabrata (25%), C.parapsilosis (20%) and C.tropicalis (11%).
83.8% C. albicans produced biofilms in comparison to 76% Nonalbicans species. 83.8% C.albicans >80% C. parapsilosis >77.7% C.tropicalis >75% C.glabrata >71.4% C. krusei exhibited adherence activity. Maximum adherence activity (4+) was shown by 32% C. albicans, 28.5% C. krusei, 25% C. glabrata, 20% C. parapsilosis and 11% C. tropicalis as shown in Table 3.
Biofilm, proteinase, and phospholipase production by Candida species isolated from clinical specimen are shown in the Table 4. All C. parapsilosis (100%) showed phospholipase activity compared to C. tropicalis (89%), C. albicans (83.9%), C. glabrata (75%) and C. krusei (42.7%) while C. parapsilosis (80%) and C. tropicalis (77.5%) were the highest proteinase producers followed by C. glabrata (75%), C. albicans (69.4%) and C. krusei (42.7%). While the adherence activity was shown by C. albicans (83.9%) and C. parapsilosis (80%) followed by C. tropicalis (77.7%), C.glabrata (75%) and C.krusei (71.4%).

Table 1: Phospholipase activity exhibited by Candida isolates
Phospholipase activity Pz<1.00 (Positive), Pz<0.7 (More virulent)
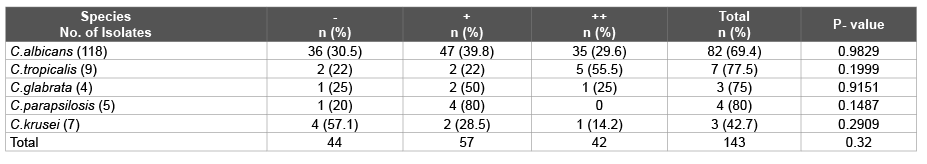
Table 2: Proteinase activity exhibited by Candida isolates (-) when no visible halo is present, (+) when visible proteolysis is limited to 1-2 mm around the colony, (++) when the zone of proteolysis >2 mm from the margin of the colony
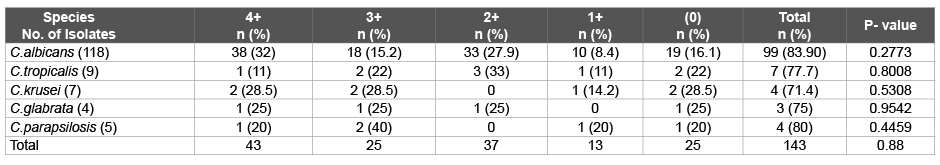
Table 3: Adherence activity exhibited by Candida isolates
4+; stongly positive, 3+ and 2+; moderately positive, 1+ and (0); weakly positive or negative.
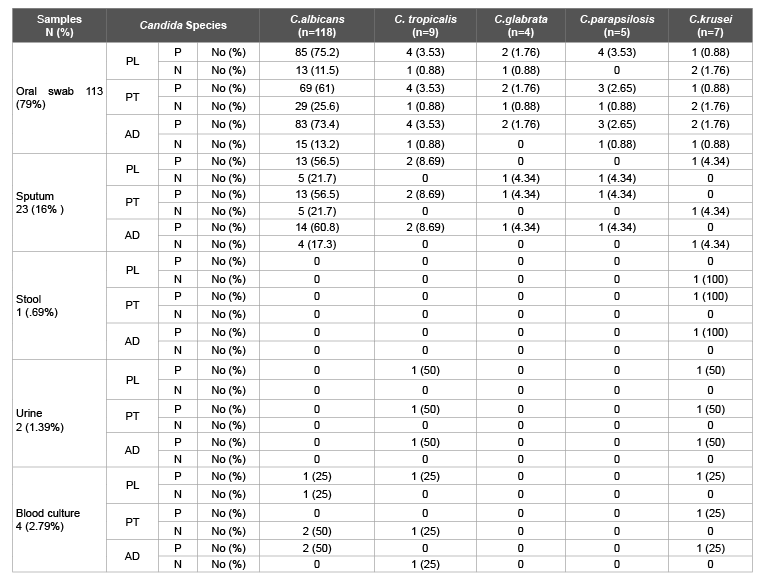
Table 4: Biofilm produced by different Candida species
Among the oral swabs: C.albicans exhibited 75%, 61%, 73% phospholipase, proteinase and adherence activity, C.tropicalis exhibited 3.5% phospholipase, proteinase and adherence activity respectively C.glabrata exhibited 1.8% phospholipase, proteinase and adherence activity and C.parapsilosis exhibited 3.5%, 2.65%, 2.65% phospholipase, proteinase and adherence activity whereas C.krusei exhibited 0.88%, 0.88%, 1.8% phospholipase, proteinase and adherence activity.
In the sputum samples C.albicans exhibited 56% phospholipase, proteinase and 61 adherence activity, and C.tropicalis exhibited 8.7% phospholipase, proteinase and adherence activity respectively. C.glabrata exhibited 4.35% proteinase and adherence activity, among C.parapsilosis exhibited 4.35% proteinase and adherence activity however in C.krusei only 4.35% detected as phospholipase positive.
In the Urine samples C.tropicalis exhibited 50% phospholipase, proteinase and adherence activity, and C.krusei exhibited 50% phospholipase, proteinase and adherence activity respectively. However, in blood culture samples C.albicans exhibited 25%, 50% phospholipase and adherence activity. C.tropicalis exhibited 25% phospholipase activity and C.krusei exhibited 25% phospholipase, proteinase and adherence activity respectively. Among the stool samples only C.krusei produced 100% proteinase and adherence activity respectively. Figure 1 represents the phospholipase, proteinase and biofilm produced by Candida species.
All the Candida spp. showed virulence activity. However, the values were not statistically significant, only the phospholipase activity by C.krusei showed statistically significant value (p=0.01). Figure 2 shows the correlation of CD4 count with different grades of phospholipase, proteinase and adherence activity by Candida spp. isolates. A positive correlation coefficient was present between CD4 count and adherence activity while a negative correlation coefficient was present between CD4 count and phospholipase and proteinase activity by Candida spp. isolates.
The pathogenicity of the species of Candida isolates is a result of the characteristics of the strains, the immunological status of the host and the local conditions of the sites of infection [11]. The pathogenicity of C.albicans is a complex process, involving different stages, colonization, adherence, invasion of tissues and damage to cells of the host, composition of the cell wall and the production of toxins and proteolytic enzymes [11,12].
Candida is an asexual, diploid, dimorphic fungus that is present in humans and in their environment. Candida organisms are commensals, and to act as pathogens, interruption of normal host defenses is necessary. General risk factors for Candida infections include immunocompromised states, diabetes mellitus, and iatrogenic factors like antibiotic use, indwelling devices, intravenous drug use, and hyperalimentation fluids. Candidiasis has emerging as an alarming opportunistic disease in the increasing in number of patients who are immunocompromised, aged, receiving prolonged antibacterial and aggressive cancer chemotherapy or undergoing invasive surgical procedures and organ transplantation.
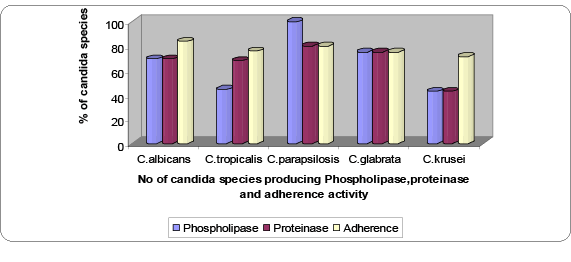
Figure 1: Phospholipase, proteinase and biofilm produced by Candida species
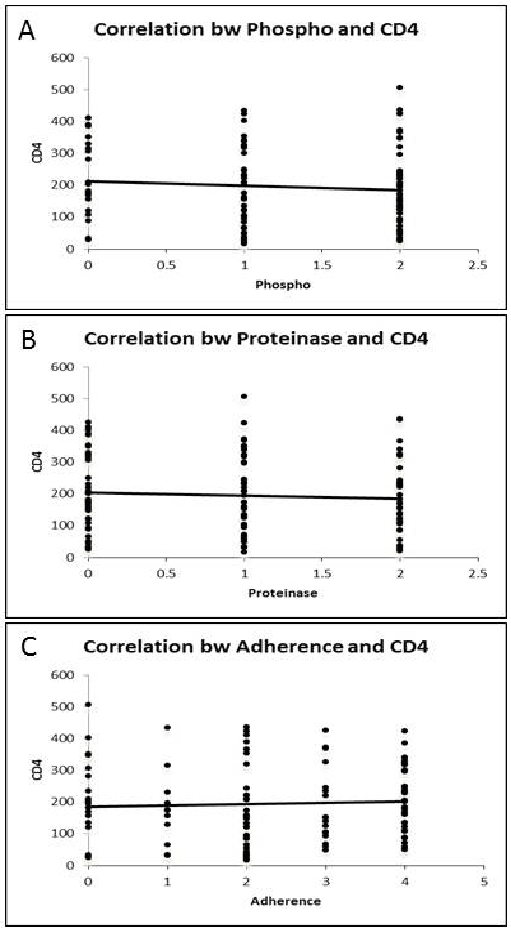
Figure 2:A) Correlation between Phospholipase and CD4 count; B) Correlation between Proteinase and CD4 count; C) Correlation between Adherence and CD4 count.
Phospholipases and aspartyl proteinases of C.albicans are considered important virulence factors [13], the absence or lowered expression of these enzymes may indicate the less virulent nature of Candida species, when compared with Candida species with higher expression of these enzymes [14,15].
Aspartyl proteinases are secreted by pathogenic species of Candida in vivo during infection. Proteinases fulfill a number of specialized functions during the infective process, they include digesting molecules for nutrient acquisition, digesting or distorting host cell membranes to facilitate adhesion and tissue invasion, digesting cells and molecules of the host immune system to avoid or resist antimicrobial attack by the host. Gokce G et al. [16] and Wu et al. [4] from Hong Kong have reported earlier that C.albicans isolates from patients with HIV infection have been known to be significantly more proteolytic (100%) than those originating from HIV-negative individuals (56%).
In our study the proteinase-producing capacity of C.albicans (69.4%) was nearly the same as of Candida nonalbicans (68%) unlike a study from Turkey in 2011 [17] which reported that C.albicans (89.7%) produced more proteinase in comparison to Candida non-albicans species (25.8%), while workers from South India in 2011 [18] reported proteinase production capacity of Candida nonalbicans (50.45%) to be less than that of C.albicans (67.34%). However a study from Italy in 2012 [19] reported that 48% C.albicans had proteinase activity while Candida nonalbicans produced no proteinases.
In our study 80% C.parapsilosis >77% C.tropicalis >75% C.glabrata >61.1% C.albicans >57.4% C.krusei exhibited proteinase activity. Highest proteinase avtivity (++) was seen in 55.5% C.tropicalis, 29.6% C.albicans, 25% C.glabrata and 14.2% C.krusei whereas 80% C.parapsilosis showed (+) activity. Among the oral swabs, 61% C.albicans, 3.5% C.tropicalis, 2.65% C.parapsilosis, 1.8% C.glabrata, and 0.88% C.krusei, exhibited proteinase activity. In the sputum isolates also similar proteinase activity was seen, 56% C.albicans, 8.7% C.tropicalis, 4.35% each of C.glabrata and C.parapsilosis. While in Urine isolates 50% each of C.tropicalis and C.krusei exhibited much more proteinase activity. In blood culture samples C.krusei exhibited 25% proteinase activity. Among the stool samples all C.krusei (100%) showed proteinase activity.
The secretion of phospholipases by C.albicans was first detected by Costa et al. [20]. C.albicans was the only Candida species known to secrete phospholipases [10,21]. Extracellular phospholipases are thought to contribute to virulence by lysing host cells or altering their surface characteristics such that adherence and penetration are facilitated [22]. Phospholipases may enhance adhesion to and cause lysis of host cell membranes. The term phospholipases refers to a heterogeneous group of enzymes that share the ability to hydrolyze one or more ester linkage in glycerophospholipids. Since phospholipase targets membrane phospholipids and digests these components, leading to cell lysis; [23] direct host cell damage and lysis has been proposed as a major mechanism contributing to microbial virulence. In our study 83.8% C.albicans produced phospholipase activity in comparison to 76% Candida nonalbicans. Also workers from Mohandas V et al. [18] showed 46.93% C.albicans producing phospholipase more than 42% Candida nonalbicans proving that C.albicans had greater extracellular phospholipase activity. The results in our study also corroborate with the reports of Ibrahim et al. [22], and Gokce G [16] from Turkey in 2007, reporting phospholipase production in 60.3% C.albicans compared to all negative Candida non albicans strains.
According to Samaranayake et al. [10], the production of phospholipase by C.albicans in vitro decreases with high concentrations of sucrose and galactose, where it is completely inhibited by a high concentration of glucose. In clinical terms, an elevated intra-oral concentration of dietary sugars could suppress the formation of phospholipase, thereby reducing the pathogenic potential of this yeast.
In our study 100% C.parapsilosis >89% C.tropicalis >83.9% C.albicans >75% C.glabrata >42.8% C.krusei exhibited phospholipase activity. The highest ++ phospholipase activity (Pz <0.7) was seen in 60% C.parapsilosis, 50% C.glabrata, 48.3% C.albicans and 44.4% C.tropicalis compared to a low percentage of C.krusei (14.2%). Previous studies have reported phospholipase activity in 30 to 100% of Candidal isolates from various groups of patients and from various sites [4,24]. Price et al. [24] reported that the proportion may depend on the site; for example, phospholipase activity has been found in 55% and 30% of Candida species isolated from blood and urine respectively whereas in our study in blood culture and urine samples, 75% and 100% of Candida species produced phospholipase. In our patients, phospholipase activity was seen in 84.7% oral Candida isolates whereas Kothavede and panthaki [25] in 1998 from Bombay showed 67.1% phospholipase activity from oral swabs whereas a study from Sri Lanka [10] reported 79% phospholipase production in isolates from oral cavity. Ribeiro et al. [26] worked on 239 oral and vaginal C.albicans strains from HIV positive patients and found a significantly higher phospholipase activity quantitatively than from HIV negative individuals.
Two publications documented, [14] that C.albicans isolates from respiratory tract infections produce larger amounts of Phospholipase than those from blood whereas in our study phospholipase production in blood samples was higher than respiratory samples but as the number of blood samples is relatively low in our study no definite conclusion could be drown. These data indicate that the isolation site of C.albicans as well as disease state of the patients may be an important factor indicating phospholipase activity. Phospholipase production is higher in urine samples (100%) than other clinical samples in our study
Biofilm and resistance development is a complex multifactorial phenomenon which still remains to be fully elucidated and understood. Different mechanisms may be responsible for the intrinsic resistance of Candida biofilms. These include the following: (i) the high density of cells within the biofilm; (ii) the effects of the biofilm matrix; (iii) decreased growth rate and nutrient limitation; (iv) the expression of resistance genes, particularly those encoding efflux pumps; and (v) the presence of “persister” cells [27]. Biofilms are a collection of microorganisms surrounded by the slime they secrete. Biofilm is presumed to promote persistence of infection. We investigated biofilms in Candida isolates of OPC patients with HIV infection and it was seen that 83.5% C.albicans and 76% Candida nonalbicans produced biofilms. Sara Asticcioli from Italy reported in 2007 a similar finding that Candida nonalbicans produced significantly less biofilms than more pathogenic C.albicans associated with increased rates of end-organ damage and a higher attributable mortality than other species, on the contrary Gokce G et al. [16] from Turkey in 2007 reported 11.8% C. albicans and 41.93% Candida non-albicans strains to be biofilm positive.
Hawser and Douglas [28] from UK in leukemic patients also reported that isolates of C.parapsilosis and C.glabrata were significantly less likely to produce biofilms than more pathogenic C.albicans. In our study, 83.8% C.albicans >80% C.parapsilosis >77.7% C.tropicalis >75% C.glabrata >71.4% C.krusei exhibited adherence activity. Maximum adherence activity (4+) was shown by 32% C.albicans, 28.5% C.krusei, 25% C.glabrata, 20% C.parapsilosis, 11% C.tropicalis. Among the oral isolates: 73% C.albicans, 3.5% C.tropicalis, 2.65% C.parapsilosis, 1.8% each of C.glabrata and C.krusei exhibited adherence activity whereas a study from Pune Maharastra in 2013 [29] on HIV/AIDS patients reported 59.6% C.albicans isolates and 73.9% Candida non-albicans from oral cavity showed biofilm positivity.
In the sputum samples also 61% C.albicans, 8.7% C.tropicalis, 4.35% each of C.glabrata and C.parapsilosis exhibited adherence activity. While in the Urine samples, 50% each of C.tropicalis and C.krusei exhibited adherence activity and in blood culture samples, 50% C.albicans and 25% C.krusei exhibited adherence activity in our study whereas a study from Karnataka India 2012 on blood stream infection in neonates, reported the occurrence of 43.75% C.glabrata, 37.5% C.tropicalis and 6.25% C.krusei positive for adherence [17]. 100% C.krusei from stool showing adherence activity produced in our study
Enhanced biofilm could potentially be an independent risk factor for prolonged disease and bad outcome. Biofilm production in this study was more related to the species of Candida than to the site of infection. Biofilm is a specific trait of a Candida isolate that is not associated with differences in adherence but with slow growth. Candida biofilms may contribute both to the pathogenesis of superficial and systemic Candidiasis as they are notoriously resistant to antifungal drugs. In this study we used SDB medium that contained high glucose (8%) which has been used to induce biofilm formation by C.parapsilosis isolates reported in several studies [30-32]. However other studies suggest that high glucose conditions, mimicking those found in TPN solutions, do not promote biofilm production by C.albicans isolates.
All the virulence markers correlated well in our study with their pathogenic potential in Candida isolates in the causation of different disease entities. The present study showed predominance of Candida parapsilosis and Candida albicans in different virulence activities, Candida parapsilosis were the maximum phospholipase produces compared to C.tropicalis, C.albicans, C.glabrata and C.krusei while, C.parapsilosis along with C.tropicalis were highest proteinase producers followed by C.glabrata, C.albicans and C.krusei. The maximum adherence activity was however seen in C.albicans followed by C.parapsilosis. The overall number of Candida albicans producing proteinase, phospholipase and biofilms are much more than the number of Candida Non albicans producing these virulence factors. The biofilm production was seen to be playing an important part for Candida albicans strains in their virulence activities while Candida Non- albicans seem to also possess other mechanisms to establish infections. There were however no significant differences in biofilm production when grouping the strains according to the patients age, and site of infection.
The mechanisms involved in the pathogenicity of different Candida species needs to be better understood from more diversified research, which would permit a greater understanding of the relations of these opportunistic parasites with their host. Also the determination of the effectiveness of different antifungal agents against the setting of biofilms has important clinical implications for guiding therapeutic decisions that potentially may affect the outcomes of patients suffering from these difficult-to-treat infections, virulence of Candida species being not attributable to a single factor but to a combination of several factors, like proteinase, phospholipase, biofilm production.
Download Provisional PDF Here
Article Type: Research Article
Citation: Kaur R, Goyal R, Dhakad MS, Bhalla P, Diwan R (2015) A Study of Virulence Factors: Proteinase, Phospholipase, and Biofilm in HIV/ AIDS Patients. J HIV AIDS 1(2): http://dx.doi. org/10.16966/2380-5536.110
Copyright: © 2015 Kaur R, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Publication history:
All Sci Forschen Journals are Open Access