Case Report
We report a case of 81 year-old man with implantable cardioverterdefibrillator
(ICD) admitted to our institution after repetitive DC-shocks
of the device. The patient was followed by our Heart Failure Center
because he had non-ischemic dilated cardiomyopathy (ejection fraction
of the left ventricle was 30%, end diastolic volume was 176 ml). 13 months
before, he was admitted to the Cardiology Department because he
developed a complete atrioventricular block and he received in primary
prevention a dual chamber ICD system (Medtronic Protecta DR; dual
coil lead 6944 Sprint 4 Medtronic for right ventricle and 5076 Capsure
fix Medtronic for the atrium). Defibrillation threshold (DFT) was tested
at the implant session and the ICD successfully terminated induced
ventricular fibrillation with a 21-Joules shock in 2 consecutive tests. The
vector configuration of shock was distal coil (RV) as cathode vs. device
can and proximal coil (SVC) as anode.
Thereafter, the patient developed relapsing pocket infection requiring
multiple surgical revisions, leading to a rotation flap and to an “over
breast” pocket which was performed with the help of a plastic surgeon
(Figure 1, Panel A). He also developed severe renal function impairment
(creatinine level 7.62 mg/dl), requiring weekly hemodialysis from a
permanent central venous catheter positioned in the right jugular vein as
it was not possible to perform an arterio-venous fistula (Figure 1, Panel
A). For these reasons, despite the relapsing infections, it was impossible a
new implant contralateral.
All of this went on for approximately 12 months, until after intense
fatigue in the garden the patient had a syncope, which was promptly
interrupted by several shocks from the ICD, resulting in an emergency
alert. The ECG performed at patient’s house (only 1 derivation) revealed
repetitive episodes of ventricular fibrillation (VF), which continued
causing several consecutive ICD’s intervention. He was treated at home
with 12 mg of midazolam iv and admitted to our cardiologic intensive
care unit. His vital signs were: blood pressure 138/70 mmHg; oxygen
saturation 98% on room air; heart rate 70 bpm. Physical examination was
unremarkable; with no signs of cardiac failure. Acid-base and electrolytic
status (in particular calcium and potassium) were normal. The ECG
showed sinus rhythm and VDD stimulation with complete AV block. The
ventricular complex had right bundle branch block morphology (Figure
1, Panel B) despite the echocardiogram showed the tip of the catheter in
the distal apex of severely dilated right ventricle. Most likely the tip of
the right ventricular lead projected to the left side due to the severe right
ventricular dilatation.
The following ICD control revealed 72 DC shocks (47 ineffective)
delivered in a few hours by the device for 32 ventricular arrhythmic
episodes interpreted by the device as VF. Nearly 50% of arrhythmic
episodes were not visible for memory saturation of device. The analysis
of latest episodes showed that the ICD correctly detected the arrhythmias
but failed to interrupt it despite a high energy shock. The first noncommitted
shock failed to interrupt VF, instead it caused a cycle length
with different morphology modification of the arrhythmia and his
spontaneous termination mean time that the device was recharging for
a second committed shock that, delivered during sinus rhythm induced
the arrhythmia again (Figure 1, Panel C) with a sequence of repetitive
shock. The other findings of the ICD were normal: battery voltage 2.7 V;
atrial sensing 1 mV; ventricular sensing 10 mV; atrial threshold 0.5 V with
0.4 ms and ventricular threshold 0.5V with 0.4 ms, normal values of lead
impedance and shock impedance.
ECG monitoring was started, as well as infusion of amiodarone and
antero-posterior external defibrillator patches were applied to the chest
as safety backups.
It is infrequent that high energy shocks for termination of ventricular
arrhythmias are ineffective, unless ICD dysfunction. We proved the absence
of any detectable dysfunction of our device, i.e. normal sensing and pacing
parameters both atrial and ventricular, acceptable shock impedance.
Several other factors may have played a role in increase DFT, however,
many could be excluded such as clinical or metabolic derangements,
pneumothorax or modification in intrathoracic impedance, and use of
antiarrhythmic agents. The location of the generator and the shock vector
can also affect the DFT, as uniform distribution of energy encompassing
the entire left ventricle is crucial [1]. Shocks from an ICD are delivered
from the coils of the lead that reach the generator by traversing through
a critical portion of the myocardium enough to break the global wave of
fibrillation. We believe that the shock’s failure in the case described was
caused by both poor can location (the can in supramammarian region
was very close to RV coil) and shock vector (coil RV vs. can) involving
insufficient mass of the left ventricle to stop the fibrillatory activation
fronts.
Management of high DFT may require both non invasive and/or
invasive strategies. Mainigi et al. [2] suggested a management algorithm
for patients with a high defibrillation threshold and failure of the initial
and maximal output shocks.
In our patient, the venography demonstrated left venous access
patency, still but we decided a conservative approach considered all
the concomitant comorbidity active. There was a chance to avoid reintervention:
altering the shock vector. Accordingly, we programmed
shocking circuit with current pathway from the RV coil to the slightly more
proximal coil (SVC coil) excluding can from the circuit. The patient was
then taken to the electrophysiologic laboratory and, previous sedation with
midazolam and fentanyl, we induced a VF by delivering direct continuous
current. The ICD successfully terminated induced ventricular fibrillation
with a 25-Joules shock, in 2 consecutive tests (Figure 1, Panel D).
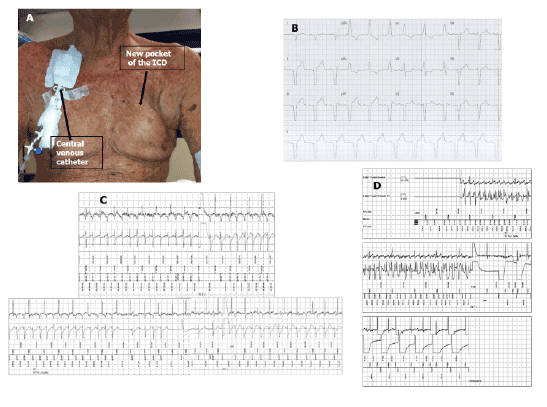
Figure 1: Panel A: An “over breast” ICD pocket is visible in the right side. A permanent central venous catheter positioned in the right jugular vein is visible in
the left side.
Panel B: 12 lead ECG of VDD pacing with RBBB morphology.
Panel C: In the upper part the first non-committed shock failed to interrupt VF. In the lower part cycle length and morphology modification of arrhythmia
and his spontaneous termination whether the device was charging capacitors for a second committed shock that, delivered during sinus rhythm
conditioned the arrhythmia’s restart.
Panel D: During DFT-ventricular fibrillation (VF) induced with erogation of direct current: the ICD successfully terminated induced VF with a 25-Joules
shock.
At the discharge the patient was asymptomatic, and remained on
amiodarone per os. 10 months later, the patient had another episode of
ventricular fibrillation during sleep correctly detected and terminated with
35-Joules DC-shock form ICD.
Conclusion
In conclusion, inefficient shock from ICD is the result of a complex
interplay between molecular, electrical, mechanical, anatomical, and
pharmacological factors. Although there have been reports suggesting
that DFT testing does not predict survival or improve clinical outcomes
in ICD recipients, there is no clear consensus about steering away from
this convention [3]. In ICD recipients who undergo device’s reposition
in alternative and non conventional sites DFT should be taken into
consideration as delivered shock could not involve critical mass and
therefore could be ineffective in solving ventricular arrhythmias. Changing
shock vector may safely and non-invasively help to manage this problem.
Download Provisional PDF Here
Article Information
Aritcle Type: Case Report
Citation: Bertini M, Marcantoni L, Groccia R,
Casadei F, Malagu M, et al. (2015) Non-invasive
Management of High Defibrillation Threshold
in Patient with Implantable CardioverterDefibrillator.
J Hear Health 1 (4): doi http://dx.doi.
org/10.16966/2379-769X.115
Copyright: © 2015 Bertini M, et al. This is an
open-access article distributed under the terms
of the Creative Commons Attribution License,
which permits unrestricted use, distribution, and
reproduction in any medium, provided the original
author and source are credited.
Publication history:
Received date: 12 October 2015
Accepted date: 24 Nov 2015
Published date: 27 Nov 2015


