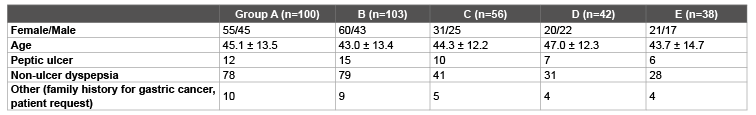
Table 1: Comparison of the demographic and clinical characteristics of the treatment groups.
Orhan Sezgin1* Mehmet Kasım Aydın1 Fehmi Ateş1 Engin Altıntaş1 Enver Üçbilek1 Serkan Yaraş1 Mahmut Bakır Koyuncu2 Gülhan Örekici Temel3
1Department of Gastroenterology, Mersin University School of Medicine, Mersin, Turkey*Corresponding author: Orhan Sezgin, Department of Gastroenterology, Mersin University School of Medicine, Mersin, Turkey, E-mail: drorhansezgin@gmail.com
Aim: We demonstrated a decade ago that standard triple therapy was unsuccessful in Helicobacter pylori (H.pylori) eradication in our region. Unfortunately, the eradication studies we have done with different therapy charts so far have proved insufficient as well. Thus, we aimed to compare combinations containing rifaximin with or without N-acetyl cysteine (NAC) with standard triple and bismuth based quadruple therapy in a prospective randomized study with the purpose of increasing eradication success.
Materials-Methods: Patients who were infected with H.pylori were included in the study. The patients were separated into 5 groups according to eradication treatments: Group A: RAC (rabeprazole 2 × 20 mg, amoxicillin 2 × 1000 mg, clarithromycin 2 × 500 mg), Group B: RAM (rabeprazole 2 × 20 mg, amoxicillin 2 × 1000 mg, metronidazole 3 × 500 mg), Group C: RBTM: (rabeprazole 2 × 20 mg, bismuth subcitrate 4 × 300 mg, tetracyclin 4 × 500 mg, metronidazole 3 × 500 mg), Group D: RARX (rabeprazole 2 × 20 mg, amoxicillin 2 × 1000 mg, rifaximin 2 × 400 mg), Group E: RARXNAS (rabeprazole 2 × 20 mg, amoxicillin 2 × 1000 mg, rifaximin 2 × 400 mg, N-acetyl cysteine 2 × 600 mg). The treatments were applied for 14 days. At least 4 weeks after the completion of treatment, eradication was confirmed by the urea breath test.
Results: A total of 339 patients were included in the study. 303 patients completed the study (89%). The eradication rates were determined to be 54% and 59% for Group A, 48% and 55% for Group B, 52% and 59% for Group C, 39% and 43% for Group D, 37% and 38% for Group E, as intention-to-treat (ITT) and per-protocol (PP) respectively. There were no significant side effects.
Conclusion: It was observed that eradication results of classical triple and quadruple therapy schedules were once again quite insufficient. Therapy regimens containing rifaximin, however, had worse results, even when NAC was added.
H. pylori; Eradication treatment; Rifaximin
Eradication criteria for H.pylori are indicated in Maastricht Consensus Guidelines. However, today’s problem is how to effectively treat patients with treatment indications. Although many treatment regimens are recommended for H.pylori treatment, an optimal one has not been identified yet [1].
H.pylori treatment has continued to be a challenging clinical problem during the last 25 years despite extensive research. H.pylori treatment with two antibiotics and proton pump inhibitors (PPI) or bismuth-containing drugs used for 7-14 days are recommended as the most effective treatment regimen [2-4]. In our country, similar to many other countries, H.pylori eradication rate with a standard triple therapy has decreased gradually due to growing antibiotic resistance (45-60%) [5-8].
Following the decline in the success rates of the traditional triple therapy, there have been attempts to develop new eradication regimens. Sequential therapy, quadruple therapy, the hybrid treatment, bismuthbased quadruple therapy regimens have been used so far. Unfortunately, these regimens had unsuccessful results as well. Researches to find a successful eradication therapy are advancing rapidly.
Rifamycin derivatives like rifampicin, rifabutine and rifaximin are the conventional drugs that have antibacterial activity against H.pylori [9,10]. Rifaximin is absorbed from the intestinal tract weakly, and it has almost no side effects. It has a very high bioavailability in the gastrointestinal tract, and when used with a colloidal bismuth subcitrate and amoxicillin, is capable of inhibiting the growth of H.pylori with an average minimum inhibitory concentration (MIC) value [11].
We thought that, decline in the eradication success rates for H.pylori treatment could be enhanced with treatment regimens which contain rifaximin with low antibiotic resistance. Therefore, our aim was to investigate the efficacy of treatment regimens with rifaximin while comparing it with standard triple and quadruple treatment protocols used for H.pylori eradication. Whether the efficacy of treatment was increased by the addition of NAC or not was tested, as well.
After local ethics committee approved the study, patients who were seen in gastroenterology clinic between January 2012 and August 2013 with dyspeptic symptoms, patients who needed to receive H.pylori eradication treatment due to peptic ulcer, patients with non-ulcer dyspepsia with H.pylori positivity on endoscopic biopsy and those who received H.pylori eradication treatment for the first time were chosen. Patients who volunteered to participate in the study signed the consent form, and they were separated into five different eradication treatment groups randomly and prospectively.
Group A: RAC (rabeprazole 2 × 20 milligrams (mg), amoxicillin 2 × 1000 mg, clarithromycin 2 × 500 mg)
Group B: RAM (rabeprazole 2 × 20 mg, amoxicillin 2 × 1000 mg, metronidazole 3 × 500 mg)
Group C: RBTM: (rabeprazole 2 × 20 mg, bismuth subcitrate 4 × 300 mg, tetracycline 4 × 500 mg, metronidazole 3 × 500 mg)
Group D: RARX (rabeprazole 2 × 20 mg, amoxicillin 2 × 1000 mg, rifaximin 2 × 400 mg)
Group E: RARXNAS (rabeprazole 2 × 20 mg, amoxicillin 2 × 1000 mg, rifaximin 2 × 400 mg, N-acetyl cysteine 2 × 600 mg). Treatment continued for 14 days for all groups.
C-14 urea breath test was performed on patients 4 to 6 weeks after the completion of 14-days treatment. Those with negative test results were regarded as successfully treated. In order to avoid false negative results in C-14 urea test, patients were strictly warned not to use PPI, H2 receptor blockers, antibiotics, and bismuth salts for 2 weeks prior to the eradication control test. Patients also reported the side effects during treatment.
Patients aged between 18-70 who needed to receive H.pylori eradication treatment according to Maastricht III consensus report, which was given as a result of clinical, laboratory and histopathological diagnosis or H.pylori positive peptic ulcer, gastropathy, duodenopathy and other conditions were included in the study.
Patients under 18 and over 70 years of age, who had pyloric stenosis, surgical history for gastrointestinal system, previous peptic ulcer disease, malignancy, history of liver or renal failure; those who were diagnosed with gastro esophageal reflux and received treatment and pregnant women or nursing mothers were excluded from the study. Patients who used H2 receptor antagonist (H2RA), prostaglandin or prokinetic agents one week before the eradication treatment started and those who were using PPI, antibiotics and agents containing bismuth salt were also not included in the study.
During upper gastrointestinal endoscopy, a total of 2 mucosal biopsies were taken from the gastric antrum and corpus from each patient. Sections obtained from biopsy samples were stained with hematoxylineosin (HE) for histological diagnosis and were stained with Giemsa for H.pylori. H.pylori infection in the light microscope was identified by the appearance of the spiral, spring-shaped bacteria. The evaluation of histological examination of tissue samples in HE stained preparations were made according to Sydney classification which was generated on the basis of clinical, morphological and etiological criteria for gastritis classification. According to that, inflammation and activity were graded as none, mild, moderate, severe and atrophy and metaplasia were evaluated as yes, no.
The results coming from the pathology laboratory were put in statistical evaluations as “H.pylori infection exists” or “H.pylori infection does not exist”. Four to six weeks after the completion of eradication treatment, C-14 urea breath test (Heliprobe, Kibion AB Uppsala, Sweden) was performed.
All patients were evaluated with ITT and PP analyses. For the statistical analysis of the data, SPSS® 11.5 statistical package program was used. As descriptive statistics number (n) and percentage (%) are given for categorical variables and for continuous variables, the mean and standard deviation are given. ANOVA was used to evaluate the differences in the age variable between the two groups. P<0.05 was accepted as statistically significant. Z test was used to control whether there is a difference between the two groups (A-B, A-C…) for ITT and PP or not. MedCalc® v.10.3 package program was used in statistical analysis.
A total of 339 patients were included in the study, out of which 152 were male (44%) and 187 were female (56%). The mean age was 45 ± 13 years. Characteristics of the patients are listed in table 1. There were no statistical differences in age, gender and the cause of H.pylori eradication treatment among the subject groups. Of these patients, 303 (89.3%) completed the study. Eradication rate was calculated according to the ITT and PP analyses. Thirty-six patients who left the study because of side effects or whose urea breath test results could not be achieved were excluded from the PP analysis, and PP analysis was carried out on 303 patients. Eradication results are given in table 2. There were no statistically significant differences in ITT ratios of the patient groups (p>0, 05). On the other hand, the difference in PP ratios was statistically significant only between Group A and E (p<0.05) and the difference among other groups was not statistically significant. In the PP analyses, the highest eradication ratio was detected in Group A (RAC) (54%), and the lowest eradication ratio was in Group E (RARXNAS) (37%). The difference was not statistically significant (p>0.05). Total eradication rate of the study groups was calculated as 47% on the ITT and 52.8% on PP.

Table 1: Comparison of the demographic and clinical characteristics of the treatment groups.
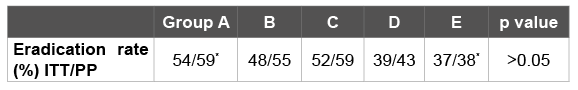
Table 2: Eradication results of the groups. *p<0.05 between group A and E in PP analysis.
Treatment compliance was very high (90%).
Side effects were seen in 8 subjects; 6 of these had received standard triple therapy. In this group, 2 patients developed skin itching, macular lesions in arms and legs. Two patients had nausea, vomiting, diarrhea, and 2 patients complained of metallic taste in the mouth and nausea. Treatment was stopped in these patients. In the other 2 patients who were receiving Group C and E treatment regimens; itching of the skin and mild allergic reaction which caused redness and macular lesions occurred, and their treatments were stopped. Side effects were mild to moderate levels, and a serious reaction was not detected. Side effect ratio was the highest in Group A and was detected as 6%. Side effect ratio was 0.5% in Groups C and E. No side effects were observed in Group B and D.
H.pylori is a gram-negative bacteria colonizing the human gastric mucosa, causing peptic ulcers, low-grade mucosa-associated lymphoid tissue lymphoma and stomach cancer, and, in these cases, it should be eradicated [12].
PPI, amoxicillin and clarithromycin/metronidazole-containing triple therapy, is generally accepted as the standard first-line treatment [13].
However, there was a significant decline in recent H.pylori eradication rates. The results of the currently used H.pylori eradication regimens were disappointing [14,15].
Although the most important reason for the failure of eradication was antibiotic resistance, other reasons included; non-compliance with the treatment, short-term treatment, side effects, drug-induced, bacterial load, smoking, and underlying concomitant diseases [16,17].
Primary resistance developing against clarithromycin and metronidazole, significantly reduced the effectiveness of eradication therapy [18].
In two previous large studies, eradication rate with standard triple treatment was calculated as 77% [19,20]. This condition was confirmed with 2 meta-analyses which were conducted on over 53.000 patients [21]. In a 10-year-long evaluation study of Ozden et al. [22], from Turkey, eradication rate was found to be 74% with 14 days long standard triple treatment. In another analyses conducted by Kadayıfçı et al. [23] in which they evaluated 94 studies that were carried out throughout a decade with a total of 3637 patients , total eradication rate was found to be 68.8%.
In another study of our group in Mersin region, eradication success rate was found to be 45% with standard triple treatment and 60% with bismuth containing quadruple therapy [8,24].
On the other hand, in this study, H.pylori eradication ratio with standard triple treatment was calculated as 54%. This study conducted in the same region after about 10 years showed us that the success of standard triple treatment is still below the aimed level.
Although we did not evaluate the antibiotic resistance in this study, we know from our previous studies that clarithromycin resistance in our region is 40% [6].
Such low eradication rates in these two studies carried out in the same region in 10 years interval might be related with high resistance against clarithromycin and virulence factors of H.pylori strains.
In our present study, eradication rate in metronidazole containing triple therapy combination (Group B) was found to be 48%. Moreover, with bismuth-based quadruple treatment (Group C) eradication rate was found to be 52%. Although there are studies suggesting that higher eradication success could be achieved with quadruple treatment combination in which bismuth is added for resistant strains against metronidazole; the efficacy of treatment regimens containing metronidazole is decreased in the presence of metronidazole-resistant strains of H.pylori [25,26].
In H.pylori treatment guides, bismuth containing quadruple treatment is requested for high clarithromycin resistance fields (more than 15%). In our study, however, the success rate of bismuth based quadruple treatment is far behind the success of standard triple treatment.
Rifaximin, an oral antibiotic that belongs to the Rifabutin family, can be highly concentrated in gastrointestinal tract as it is not absorbed by the stomach and intestinal mucosa. It has a broad spectrum against grampositive or gram-negative enteric bacteria (in vitro activity) [27]. MIC (minimum inhibitory concentration) value of rifaximin can be compared with many antibiotics used for H.pylori eradication. As it is not absorbed, it has a low risk of causing resistance [28,29].
Due to the lack of bacterial resistance against the drug [10,27,29], some preliminary studies have investigated the efficacy of rifaximin for H.pylori eradication [30,31]. In one-blind randomized study, efficiency of rifaximin alone and in combination therapy in H.pylori positive patients with antral gastritis was evaluated, and it was found to be effective especially when combined with clarithromycin. In this study, with rifaximin alone 400 mg 2 × 1 40%, with rifaximin+CBS 120 mg 2 × 1 combination 50%, with rifaximin+clarithromycin 500 mg 2 × 1 combination 73% and with rifaximin+metronidazole 250 mg 2 × 1 combination 60% eradication rates were obtained [32].
Based on these results, triple therapy consisting of rifaximin, amoxicillin and omeprazole were tested. However, the result was devastating, and the eradication rate was not different from the one observed with double treatment (60%) [33].
In three pilot studies in which efficiency of triple treatment containing rifaximin was tested for H.pylori eradication, eradication rates were reported to be between 40-58% [28,32,34]. The relatively low eradication rates reported in the studies above could be related to many factors such as non-optimal dosage or the lack of synergy between the components of a therapeutic regimen. In our study, we found the eradication rate of the groups using rifaximin to be 39%. We identified that this result was at an unacceptable level, like the studies above.
H.pylori resides on the mucosal surface under the mucus layer, and insufficient concentration of rifaximin in mucus layer could affect eradication rates negatively. Thus, combination of mucolytic agents such as NAC with rifaximin may increase the effect of rifaximin via increasing the adhesion to the mucus layer [35].
Mucus has a dense and sticky character as a result of the high rate of disulfide bonds in it. NAC shows mucolytic effect on the mucoid and mucopurulent secretions because of its sulfhydryl groups that have the capability of breakout the disulfide bonds inside the mucus glycoprotein [36].
NAC, glutathione precursor, is an important protective factor against gastric mucosal damage and plays an important role in mucosal protection and healing. Intracellular antioxidant effects and toxin neutralization effects of glutathione are well known [37]. NAC can be used as a mucolytic agent with viscosity-reducing effect and by opening disulfide bonds in patients who have solid and dense mucus in the airways [38]. Because of the lowering effect of NAC on the gastric mucous viscosity, its place in H.pylori treatment was examined in a limited number of studies [39]. In a study carried out by Zala et al. [40], the same antimicrobial agents for H.pylori eradication were applied at the same dose and for the same duration in the two patient groups, and higher eradication rates were determined for patients using NAC with nicotine.
Another placebo-controlled study did not detect significant differences in the rate of eradication; eradication rate was found to be 70% in treatment group and 60% in placebo control group [41].
In our study, the eradication rate of the NAC, rifaximin combination group was 37%, which is the lowest among all groups.
In conclusion, H.pylori eradication therapy is still difficult and challenging despite all treatment attempts. Standard triple treatment, bismuth-based quadruple treatment and rifaximin combinations with and without NAC have not been successful in this study.
Download Provisional PDF Here
Article Type: Opinion Article
Citation: Sezgin O, Aydın MK, Ateş F, Altıntaş E, Üçbilek E, et al. (2016) Addition of Rifaximin and N-Acetyl Cysteine to the Standard Helicobacter pylori Treatment Regimens: Is it Possible to Improve Outcomes? J Gastric Disord Ther 2 (2): doi http:// dx.doi.org/10.16966/2381-8689.117
Copyright: © 2016 Sezgin O, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Publication history:
All Sci Forschen Journals are Open Access