
Figure 1: Average percent return of pesticides and PCBs sorbed to six microplastic polymers


Todd Allen Savannah Farley Joshua Draper Cassandra Clement Beth Polidoro*
School of Mathematics and Natural Sciences, Arizona State University, Glendale, AZ 85306, USA*Corresponding author: Beth Polidoro, School of Mathematics and Natural Sciences, Arizona State University, Glendale, AZ 85306, USA, Tel: 602-543-5686; E-mail: beth.polidoro@asu.edu
Microplastic pollution is a growing global concern across terrestrial, marine and freshwater environments. It is welldocumented that a number of organic contaminants are also associated with microplastics in the environment. In a controlled experiment, this study documents the equilibrium sorption potential of 32 persistent organic pollutants, representing different pesticides and polychlorinated biphenyls (PCBs), to the six most commonly-used plastic polymers. Results showed that sorption rates, measured as sorption recoveries or percent return, for individual contaminants varied widely. However, the plastic polymers polyvinyl chloride (PVC) and low-density polyethylene (LDPE) showed the highest average sorption recoveries for PCBs, while LDPE also showed the highest average sorption recoveries for pesticides. The lowest average sorption recoveries for both pesticides and PCBs were observed in the polymer polyethylene terephthalate (PET). Given that these contaminants have high persistence, toxicity and bioaccumulation potential, the results of this study can serve as a guide to industry, government and other stakeholders for prioritizing additional research or regulation for polymers that may pose a higher risk to aquatic ecosystems and human health, due to their higher contaminant sorption potential.
Microplastics; Contaminants; Chemical equilibrium; Polymers
Plastics manufacturing now exceeds 300 million tons annually [1,2]. With the projected longevity of plastic waste [3], there is an urgent need for a better understanding of the environmental and health-related effects of microplastic pollution. Plastic polymer manufacturing is dominated by six “commodity plastics” that include low-density polyethylene (LDPE), high-density polyethylene (HDPE), polypropylene (PP), polyvinyl chloride (PVC), polystyrene (PS), and polyethylene terephthalate (PET), [4]. Plastics in the environment are subject to harsh elemental conditions, whether through improper disposal or long-term storage in landfills, and can break down over time into small particulates, with fragments less than 5 mm in size termed microplastics [5]. Over the past decade, microplastics have been found to permeate urban and natural environments worldwide, with limited means of removal [6-9]. The ubiquity of microplastic pollution poses a major threat to marine and freshwater ecosystems [7,10,11].
It has been well documented that microplastics act as carriers of other chemicals due to their ability to sorb organic pollutants in the environment [12-14]. As a result, microplastics are thought to contribute to pollutant transport into marine organisms via inadvertent consumption, which may or may not exacerbate the risk of contaminant bioaccumulation and subsequent permeation into the human diet [15-20]. However, only a few studies [21,22], are known to have experimentally explored differences in organic contaminant sorption potential across the six main commodity plastics, which constitute the majority of manufactured plastic polymers.
Therefore, the objective of this study was to examine differences in equilibrium sorption of 32 organic pollutants (comprised of PCBs and primarily organochlorine pesticides) to microplastics derived from six common plastic polymers. Thirty of the 32 pollutants analyzed are (or have parent compounds that are) listed in the Stockholm Convention on Persistent Organic Pollutants (PoPs). PoPs under the Stockholm Convention include all PCBs and several organochlorine pesticides (aldrin, chlordane, DDT, dieldrin, endrin, heptachlor, hexchlorobenzene, mirex, toxaphene, endosulfan) which are included in this study, as well as their related isomers or degradation products (DDD, DDE, nonachlor,heptachlor epoxide, lindane, BHC alpha isomer, etc). Only two of the 32 compounds (methoxychlor and chlorpyrifos) analyzed in this study are not currently included under the Stockholm Convention. However, both of these pesticides have been proposed as candidates for inclusion given their persistence and toxicity, as well as have been banned or restricted in several countries around the globe [23]. Given that these pollutants have high persistence, toxicity and bioaccumulation potential, the results of this study, along with other studies on individual contaminant sorption [22] or mixtures of contaminant sorption [21] to plastics and microplastics, can serve as a guide to industry, government and other stakeholders for prioritizing plastics polymers that may pose a higher risk to aquatic ecosystems and human health.
Samples of new, common household plastic items were obtained for polymers labeled 1-6 in the United States: 1- polyethylene terephthalate (PET), 2- high density polyethylene (HDPE), 3-polyvinyl chloride (PVC), 4- low density polyethylene (LDPE), 5-polypropylene (PP), and 6-polystyrene (PS). A medium-fine metal file was used to create microplastic particles of each plastic type ranging in size from 0.5mm to 2 mm. Approximately 0.1 g of each microplastic sample was mixed with 10 mL of tap water. Over a series of different trials, the microplastics in water were spiked with known amounts (ranging from 10-15 µg) of 32 non-polar contaminants (19 chlorinated pesticides and metabolites and 13 PCBs), as well as a recovery surrogate (p-terphenyl). Spiked microplastic samples in water were mixed in sealed jars on a rotator for 72 hours. The microplastics were removed, and placed in 15 mL of hexane and rotated another 72 hours in sealed jars. All hexane extracts were dried with NaSO4 and concentrated to a final volume of 0.5 ml with nitrogen gas. Final extracts were spiked with the internal standard tetracosane-d50, and analyzed for the organic contaminant recovery using a Varian 3800 gas chromatograph in tandem with a Saturn 2200 electron ionization mass spectrometer. Results are reported in percent return, which was calculated as the difference between the amounts of contaminant recovered after microplastic sorption compared to the initial amount added to the microplastic/ water mix. For each trial, laboratory blanks (e.g. non-spiked microplastics) were also exposed to tap water, extracted in hexane and analyzed. Method detection limits and recoveries for all contaminants extracted from water are reported in Pulford et al. [25] and Polidoro et al. [26]. However, given that this was a pilot study, future work includes additional laboratory trials and replicates for these and other compounds. The list of 32 contaminants with their average log octanol/water partition coefficients (Kow) are shown in Table 1 [27]. A standard ANOVA and paired T-tests (assuming unequal variances) were used to test if average logs Kow were a good predictor of contaminant sorption to different plastic polymers.
| Organochlorine Pesticides | Log Kow Range* | Average Log Kow |
| BHC alpha isomer | 3.8 | 3.8 |
| Endosulfan | 3.83 | 3.8 |
| Lindane | 3.78-4.14 | 4.0 |
| DDD | 4.32 | 4.3 |
| Methoxychlor | 4.68-5.08 | 4.9 |
| Chlorpyrifos | 4.7-5.11 | 4.9 |
| Hexachlorobenzene | 4.79-5.74 | 5.3 |
| Mirex | 5.28 | 5.3 |
| Heptachlor | 5.44 | 5.4 |
| Heptachlor epoxide | 5.44 | 5.4 |
| Endrin | 5.43-5.6 | 5.5 |
| cis-Nonachlor | 5.54 | 5.5 |
| cis-Chlordane | 6.16 | 6.2 |
| trans-Chlordane | 6.2 | 6.2 |
| trans-Nonachlor | 6.08-6.44 | 6.3 |
| DDT | 6.36 | 6.4 |
| DDE | 6.51 | 6.5 |
| Dieldrin | 5.38-7.67 | 6.5 |
| Aldrin | 5.68-7.4 | 6.5 |
| Polychlorinated Biphenyls (PCBs) | ||
| Biphenyl | 3.88-4.04 | 4.0 |
| 3.5-Dichlorobiphenyl | 4.11-5.41 | 4.8 |
| 2.4.6-Trichlorobiphenyl | 5.47-5.69 | 5.6 |
| 2.2'.5.5'-Tetrachlorobiphenyl | 5.11-6.20 | 5.7 |
| 2.2'.4.5'-Tetrachlorobiphenyl | 5.81-6.34 | 6.1 |
| 2.3'.4.5'-Tetrachlorobiphenyl | 6.34-6.41 | 6.4 |
| 2.3.5.6-Tetrachlorobiphenyl | 6.43 | 6.4 |
| 3.3'.4.4'-Tetrachlorobiphenyl | 6.72 | 6.7 |
| 2.2'4.5.5'-Pentachlorobiphenyl | 6.80-6.98 | 6.9 |
| 2.3.4.4'.5-Pentachlorobiphenyl | 6.98-7.12 | 7.1 |
| 2.3'.4.4'.5-Pentachlorobiphenyl | 6.98-7.12 | 7.1 |
| 2.3.3'.4.4'.5'-Hexachlorobiphenyl | 7.62-7.44 | 7.5 |
| 2.2'.4.4'.5.5'-Hexachlorobiphenyl | 7.62-7.75 | 7.7 |
Table 1: List of 19 primarily organochlorine pesticides and 13 PCBs added to different plastic polymers, in order of increasing average log Kow. (*primarily from Schwarzenbach et al. [27])
PVC showed the highest average sorption rates or percent return for PCBs, followed by LDPE and HDPE. LDPE had the highest average sorption rates across all pesticides (Figure 1). Lowest average sorption rates across both pesticides and PCBs were observed in PET. Average percent recovery of PCB congeners in tap water ranged from 19% (PET) to more than 66% (PVC) among the different plastic polymers, while average chlorinated pesticides percent return ranged from about 11% (PET) to more than 37% (LDPE).

Figure 1: Average percent return of pesticides and PCBs sorbed to six microplastic polymers
Among individual pesticides, endrin and chlorpyrifos showed the highest percent returns (75% and 60% respectively) to PVC, and across all plastic polymers compared to other pesticides (Figure 2). Endrin’s high sorption potential may be related to its higher molecular mass relative to other pesticides, or potentially from the reactivity of oxygen in its chemical structure. Among PCB congeners, PVC consistently exhibited higher percent returns, ranging from about 60-75%, with the exception of biphenyl which showed less than 30% return (Figure 3). PET percent return rates were 22% or less across all PCB congeners. Field studies in the marine environment have also observed lower concentrations of PCBs sorbed to PET in relation to other plastic polymers, but in contrast, have also observed relatively low concentrations of PCBs sorbed to PVC [28]. With the exception of biphenyl, 3,5dichlorobiphenyl, and 2,4,6-trichlorobiphenyl there was no significant observable difference among percent returns across different PCB congeners to the same plastic polymer (Figure 3).
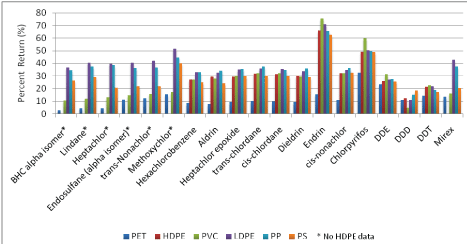
Figure 2: Percent return of 19 pesticides and metabolites across six microplastic polymers
Based on analyses of variance (ANOVA) for all contaminant sorption rates across all plastic polymer types, log Kow was a significant predictor of sorption to plastics, both within each class of compounds and across all polymer types (p<0.05). This correlation between log Kow and organic contaminant sorption to microplastics in seawater has also been found in other studies [22]. However, the rate of sorption may vary widely, even as compounds with high log Kow values likely sorb onto organic polymers more rapidly due to increased hydrophobicity [29].
In our experiment, average sorption or percent return across all combined PCBs and pesticides decreased in the order of LDPE>PVC>HDPE ≥ PP ≥ PS>PET. With the exception of PVC, these results are similar to experimentally derived plastic-water partition coefficients summarized by other studies [30,31] for a variety of PCBs and other chemicals, e.g. LDPE ≈ HDPE ≥ PP>PVC ≈ PS. In field studies conducted in marine waters, higher concentrations of PCBs and PAHs were also found in LDPE, HDPE and PP, compared to PVC and PET [28]. Compared to these other similar studies, the increased sorption of contaminants to PVC in our study may have been due to potentially increased surface area during microplastics preparation (e.g. filing), and/or decreased crystallinity due to lack of weathering.
Contaminant sorption to different plastic polymers has been shown to vary based on both chemical and physical properties, including hydrophobicity [32,33], surface area [34], size [12,13], diffusivity [33,35], and crystallinity [33]. Glassy plastic polymers, such as PET and PVC, have less diffusion and reduced sorption capacities compared to nonglassy or rubbery polymers, such as HDPE, LDPE and PP [36]. The relative higher diffusivity of LDPE may account for its popularity for use as environmental equilibrium or passive samplers for the adsorption and detection of a wide variety of organic contaminants [37,35]. Similarly, Huffer and Hofmann [38] found that linear isothermic sorption of selected PAHs to polyethelene (PE) was likely due to absorption into the bulk polymer, while non-linear isothermic sorption by PS and PVC may be due to surface adsorption.
In our study, microplastics were derived from new plastic products filed to the same size and shape. However, the size, age, wear and tear, and degradation of microplastics in the environment influence sorption rates across different polymers [39]. In general, sorption rates increase with decreasing particle size [40]. Polymer photodegradation has been shown to increase sorption capacity, through increased surface area [28,41]. Similarly, diffusion has been shown to be slower into virgin plastic materials, compared to eroded plastic that shows increased crystallinity [35].
Contaminant exposure time to microplastics is also important for maximizing plastic polymer sorption recoveries. In the laboratory, sorption of chemicals to plastics has been shown to come to equilibrium in less than 72 hours [34]. However, other laboratory studies of polyethene and polypropylene showed that equilibrium for phenanthrene ranged from 20 to more than 80 days [35]. Under field conditions, a variety of environmental factors, including biofouling, can influence contaminant equilibrium conditions, sorption and degradation. In the field, equilibrium may occur much slower, from months to more than 1 year [14,28].
Eighteen of the 19 pesticides tested are organochlorine pesticides (or are their degradation products or different isomers). Chlorpyrifos is classified as an organophosphate pesticide, but given its polarity and chlorination, chlorpyrifos environmental behavior and equilibrium partition characteristics are closer to those of organochlorine compounds [42]. Chlorinated pesticides were more commonly used before the 1970s, but are persistent in the environment and continue to pose potential environmental and health risks [43-46]. Chlorinated pesticides are characteristically highly chlorinated cyclic hydrocarbons that have distinctive properties, including insolubility in water, persistence in soil, and bioaccumulation in the adipose tissue of organisms [47]. Several of the chlorinated pesticides belonging to the cyclodienes subgroup, including aldrin, dieldrin, heptachlor epoxide, and chlordane, which demonstrated very similar patterns of sorption across the six plastic types, likely due to their similar cylcodiene structure.
PCBs are synthetic organic compounds comprised of two conjugated cyclohexane rings with a varying number of attached chlorine atoms. With 209 distinct congeners, the number of chlorine atoms and their placement within the biphenyl ring system primary determines each congener’s physical and chemical properties. As a class, PBCs exhibit low vapor pressures, low water solubility, and high dielectric constants [48]. As the degree of chlorination and subsequent molecular weight increases, the sorption of different PCBs show a slight increase (Figure 3), indicating that the number of chlorine atoms may influence PCB sorption as well as the positioning of chlorine atoms on the biphenyl rings, especially as PCBs that are congruent in composition but different in structure rendered very similar percent return. Although the effects of varying chlorine position on sorption are not yet clear, trends may be generalized by comparing sorption rates among PCB isomers [49], and it is likely that the configurations and bulkiness of individual PCB congeners would affect the sorption of different PCBs onto polymers [50].
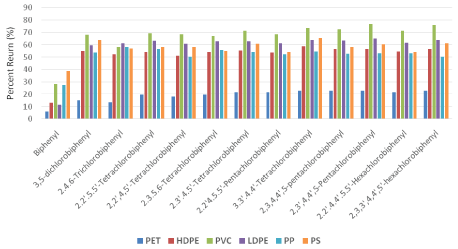
Figure 3: Percent return of 13 PCB congeners across six microplastic polymers
Our study found that PCB adsorption to microplastics was higher than that of organochlorine pesticides, given an exposure time of 72 hours in under controlled freshwater conditions. In general, average sorption or percent return across all combined PCB and organochlorine pesticides decreased in the order of LDPE>PVC>HDPE ≥ PP ≥ PS> PET. Additionally, contaminant log Kow may be a useful predictor for plastic sorption rates. However, differences in organic contaminant sorption rates to plastic polymers outside of laboratory conditions can vary widely based on a number of different environmental factors, including plastic surface area, plastic weathering rates, contaminant exposure time, contaminant degradation conditions, contaminant mixtures, and biofouling.
We thank the Arizona State University NCUIRE program for project support and funding.
Download Provisional PDF Here
Article Type: RESEARCH ARTICLE
Citation: Allen T, Farley S, Draper J, Clement C, Polidoro B (2018) Variations in Sorption of Organochlorine Pesticides and PCBs across Six Different Plastic Polymers. J Environ Toxicol Stud 2(1): dx.doi. org/10.16966/2576-6430.109
Copyright: © 2018 Allen T, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Publication history:
All Sci Forschen Journals are Open Access