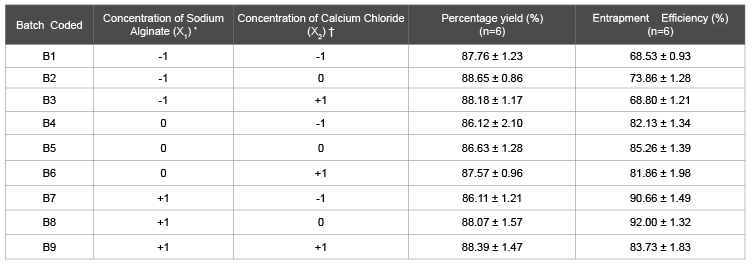
Table 1: Different batches with their composition (factor level), percent yield and entrapment efficiency
*
X1 levels [200 mg (-1), 400 mg (0), 600 mg (+1)]
†
X2
levels [0.5% (-1), 1 %( 0), 1.5 %( +1)]

Mukesh Shinde1* Prasad Gunjal1 Vikram Gharge1 Mukund Gurjar2
1Formulation and Development Department; Zuventus Healthcare Ltd, T-184 MIDC Bhosari, Pune, 411026, Maharashtra, India*Corresponding author: Mukesh Shinde, Sr. Research Fellow, Formulation and Development Department, Zuventus Healthcare Ltd, T-184 MIDC Bhosari, Pune, 411026, Maharashtra, India, E-mail: mukesh.shinde@zuventus.com
The aim of this research was to develop a multiparticulate floating drug delivery system using porous calcium silicate and sodium alginate, for site specific drug release of ranolazine. Ranolazine is an anti-anginal drug with extensive and highly variable hepatic first pass metabolism following oral administration, with systemic bio-availability of 76% and relatively short plasma half-life of 2.5 ± 0.5 hours, so there is a need for the development of prolonged release formulation for the same. The drug loaded beads were prepared by adsorbing drug on the florite, by rapid solvent evaporation and further was used to prepare calcium alginate beads by inotropic gelation method, using 32 factorial designs. Developed formulations were evaluated for yield, entrapment efficiency, image analysis, surface topography, mechanical strength, apparent density, buoyancy studies and dissolution studies. Developed formulations showed instantaneous floating with very slow drug release in acidic medium. Quantity of porous carrier and concentration of sodium alginate solution significantly affected the performance of beads. By altering the amount of these two components in formulation floating time of beads could be controlled ranging up to 6.30 h. Drug release from beads in acidic environment was influenced by both quantity of Florite RE® and sodium alginate concentration while initial drug release in simulated intestinal environment was primarily controlled by sodium alginate concentration.
Pours carrier; Calcium alginate beads; Multiparticulate floating drug delivery
Site and time specific oral drug delivery have recently been of great interest in pharmaceutical field to achieve improved therapeutic efficacy. Gastroretentive drug delivery system is an approach to prolong gastric residence time, thereby targeting site-specific drug release in upper gastrointestinal (GI) tract. Floating drug delivery system (FDDS) and bioadhesive drug delivery are the widely used techniques for gastroretention [1,2] the latter have limitation of localized high drug concentration that could lead to irritation or ulceration.
Low density porous carriers have been used by researchers for formulation of FDDS [3,4]. Porous carriers are low density solids with open or closed pore structure and provide large exposed surface area for drug loading. Their hydrophobicity varies from completely hydrophilic carriers, which immediately disperse or dissolve in water, to completely hydrophobic ones, which float on water for hours. Due to wide range of useful properties, porous carriers have been used in pharmaceuticals for many purposes; some of these includes development of novel drug delivery systems like floating drug delivery systems, sustained drug delivery systems; improvement of solubility of poorly soluble drugs; enzyme immobilization etc [5,6]. Examples of pharmaceutically exploited porous carriers include porous silicon dioxide (Sylysia® ), polypropylene foam powder (Accurel® ), porous calcium silicate (Florite® ), magnesium aluminometa silicate (Neusilin® ), porous ceramic, etc. Florite RE® (FLR) is a porous calcium silicate [2CaO·3SiO2 ·mSiO2 ·nH2 O (1<m<2, 2<n <3)], and possess a lot of pores particularly of size 0.15 µm on its surface FLR has been used to adsorb oily and other drugs, as a compressive agent in pharmaceuticals and to improve solubility [3,7,8]. The majority of drugs are preferentially absorbed from the upper part of the small intestine [9], hence, drug release at site of better absorption can improve therapeutic efficacy of drug.
Ranolazine is antianginal drug, approved by US FDA in 2006. It is marketed as extended release tablets (Ranexa 500 mg/1 gm). Ranolazine is extensively metabolized in the liver and its absorption is highly variable. The apparent terminal half-life of Ranolazine is 7 hrs. Ranolazine has relatively high solubility (42.08 mg/ml in 0.1 N HCl) at low pH in the stomach (pH 1.2 – 3). The high acid soluble property of ranolazine results in rapid drug absorption and clearance, causing large and undesirable fluctuations in plasma concentration of ranolazine and short duration of action, thus necessitating frequent oral administration for adequate treatment [10-13].
The aim of this research was to develop a multiparticulate floating drug delivery system using porous calcium silicate (Florite RE® ) and sodium alginate for site specific drug release of ranolazine. The drug loaded beads were prepared by adsorbing drug on florite (FLR) by rapid solvent evaporation and further was used to prepare calcium alginate beads by inotropic gelation method using 32 factorial designs. Developed formulations were evaluated for yield, entrapment efficiency, image analysis, surface topography, mechanical strength, apparent density, buoyancy studies and dissolution studies.
Ranolazine and Sodium alginate was utilized from Zuventus Healthcare ltd, India. Florite RE® (FLR) was gifted from Tokuyama Corporation (Yamaguchi, Japan). All other chemicals were analytical grade.
Accurately weighed quantity of ranolazine (375 mg) was dissolved in methanol in 250 ml round bottom flask followed by FLR (187.5 mg) was dispersed with shaking; subsequently methanol was evaporated under vacuum rotary evaporator. The blank and drug loaded beads were prepared by the ionotropic gelation method. The drug adsorbed FLR powder (1: 0.5 ratios) was added to 25 ml of alginate solution under stirring to get uniform dispersion. The obtained dispersion was extruded through an 18G (1.2 mm diameter) needle drop wise (4 ml/min) for 10 cm height into calcium chloride 1% solution to get gel beads which were allowed to remain in the calcium chloride solution for 30 min under stirring. The obtained beads were filtered and dried at room temperature 37°C ± 5 for 24 h. The 32 factorial design was used to study the effect of sodium alginate and calcium chloride on characteristics of drug loaded beads [14].
Calcium alginate beads containing ranolazine were prepared based on the 32 factorial design. Quantity of FLR (X1 ) uses to adsorb ranolazine and concentration of sodium alginate (X2 ) were selected as two independent variables. Three levels determined from preliminary studies of each variable were selected and nine possible batches were prepared using different levels of variables A polynomial equation (Eq. 1) was used to study the effect of variables on different evaluation responses (Y), where the coefficients in the equation (β0 , β1 , β2 , β12) were related to the effects and interactions of the factors.
Y = β0 + β1 X1 + β2 X2 + β11X21 + β22X22 + β12X1 X2 (1)
Where β0 is the arithmetic mean response of nine batches, β1 and β2 coefficients of factor X1 and X2 and β12 the coefficient of interaction of X1 and X2 . The interaction (X1 X2 ) shows how the dependent variable changes when two or more factors are simultaneously changed.
Accurately weighed quantities of beads were dissolved in 100 ml of 0.1 N HCl and filtered through membrane filter (0.45 µ). The filtrate was diluted and assayed by using spectrophotometer at 271 nm (JASCO V500, Japan).
Entrapment efficiency was calculated by,
$${\rm{Entrapment Efficiency = }}{{{\rm{Actual Drug Content in Beads}}} \over {{\rm{Theoretical Drug Content in beads}}}}{\rm{X 100}}...........{\rm{(2)}}$$
The external morphology of drug loaded beads was determined by scanning electron microscopy (Stereoscan S120, Cambridge, UK). Samples were mounted on a double faced adhesive tape and coated with thin gold-palladium layer by sputter coated unit (VG-Microtech, Uckfield, East Sussex, UK) and surface topography were analyzed at 500X. The average diameter and different shape factors such as circulatory factor and roundness of beads were determined by using a stereomicroscope (Model BH-2, Olympus, Japan).
Crushing strength of five beads from each batch was determined using mercury load cell method [14].
To determine the floating time, the beads (20) were dipped in the 900 ml of 0.1 N HCl in the USP type II dissolution apparatus (Elecrtrolab, Mumbai India) for 8 hrs under stirring (100 rpm) at 37.5 ± 0.5° C. At selected interval, stirring was stopped and the number of settled beads was counted for 2 min.
The differential scanning calorimetry thermograms of ranolazine, placebo beds and drug loaded beads, were obtained using a Mettler Toledo DSC 821e (Switzerland) instrument equipped with an intra cooler. Instrument was calibrated for DSC temperature and enthalpy using Indium standard. The samples were hermetically sealed in perforated aluminium pans and heated at constant rate of 10° C/min over the temperature range of 0-200°C. The system was purged with nitrogen gas at the rate of 100 ml/ min to maintain inert atmosphere.
The crystalline properties of pure ranolazine, blank and drug loaded beads were studied using X-ray diffractometer (PW 1729, Philips, Netherlands). The samples were irradiated with monochromatized Cu Kα radiation (1.542 Ǻ) and analyzed at 2θ between 5° to 50° . The voltage and current used were 30 kV and 30 mA respectively. The range and the chart speed were 2 × 103 CPS and 10 mm/degree 2θ, respectively.
IR spectra of drug, FLR, sodium alginate and drug loaded beads samples were obtained on Jasco V5300 Fourier transform infrared spectroscopy (FTIR) (Jasco, Tokyo, Japan). The pellets were prepared on KBr press (Spectra Lab, Mumbai, India) using mixture of sample and KBr in ~1:10 ratio. The spectra were recorded over the wave number range of 4000 to 400 cm−1.
Dissolution study of drug loaded beads was performed in the 0.1 N HCl using USP type II dissolution test apparatus (Electrolab, Mumbai, India). Drug equivalent to 100 mg of beads were placed in 900 ml of dissolution medium which was stirred with rotating paddle at 100 rpm, with temperature adjusted to 37 ± 0.2° C. At selected time intervals, samples was removed and replaced with fresh dissolution medium. The sample was then filtered through Whatman filter paper and analysed by UV spectrophotometer (JASCO-V500, Japan) at 271 nm.
Calcium alginate beads offer a simple multiparticulate drug delivery system for macromolecules and drugs because of it’s highly pH dependent characteristics of swelling and drug release [15,16]. To limit bulk volume the quantity of FLR was restricted to maximum of 0.6 g for adsorption of 375 mg of Ranolazine. Preliminary study to obtain beads was carried out using 0.1–2% (w/v) sodium alginate solution and 1–5% (w/v) calcium chloride solution as cross-linking medium. It was observed that 0.5–2% (w/v) sodium alginate solution containing 375 mg/ml of drug adsorbed FLR, extruded in 1% (w/v) calcium chloride solution produced beads with instantaneous floating ability. An attempt was also made to obtain alginate beads containing pure drug without FLR using above formulation and processing parameters but friable discs in place of spherical beads were produced. On the other hand, beads containing pure FLR were easily formed using same procedure. This indicate that hydrophilic nature of FLR causes greater contact between solid particles and sodium alginate solution needed for bead formation, whereas hydrophobic nature of ranolazine did not allowed optimum contact of drug particles and sodium alginate solution. The concentrations of sodium alginate solution used in present study were also lower than usually used by researchers (2%, w/v or above) to prepare alginate drug beads [17,18]. The yield and entrapment efficiency of different batches of beads were found in the range 86.11-88.65 and 68–92%, respectively (Table 1). Percent yield of beads was observed to increase with increase in both the quantity of FLR and concentration of sodium alginate maybe due to increased bonding and encapsulation of particles in beads.

Table 1: Different batches with their composition (factor level), percent yield and entrapment efficiency
*
X1 levels [200 mg (-1), 400 mg (0), 600 mg (+1)]
†
X2
levels [0.5% (-1), 1 %( 0), 1.5 %( +1)]
The beads of all the batches were spherical with roundness and circulatory factor close to 1.0 (Table 2). The scanning electron microscope images of beads illustrate the spherical shape with thin and incomplete calcium alginate surface coat (Figure 1). Also beads containing higher quantity of FLR were more spherical and smooth surfaced compared to beads containing lesser quantity of FLR (Figure 1), keeping alginate concentration constant. This is also supported by roundness and circulatory factor data of batch 8 beads that showed lowest circulatory factor and high roundness (Table 2). Beads with less FLR quantity showed surface deposition of ranolazine crystals which are very scanty on surface of beads containing higher quantity of FLR. The size of beads varied from 2.0 to 2.6 mm for different batches. Keeping other factor constant, the bead size was found to increase with increase in FLR quantity in formulation and decrease in concentration of sodium alginate but former showed major effect (Figure 2) as also depicted by sign and value of coefficients in (Table 3). This increase in bead size may result because of low density of FLR.
PXRD of ranolazine showed characteristic peaks at ~16.2°, 21.2°, 23.2°, and 24.5° (2θ). This Peak at 16.2° (2θ) was used to compare PXRD pattern of drug with microparticles (Figure 3). Significant reduction in peak intensities was observed in PXRD pattern of microparticles when compared with pure drug, but this reduction was not significantly different from reduction obtained in case of placebo beads. It may be attributed to dilution effect of FLR, as also indicated in DSC studies.
Thermal properties of drug, placebo beads and drug loaded beads were studied using DSC (Figure 4). Ranolazine showed sharp melting endotherm (A) at 124.63°C. DSC of placebo beads shows no endothermic (C) peak up to 200°C. Thermogram of Drug loaded (B) showed a broad endotherm ranging from 118.92°C to 122.05°C (Figure 4). Normalized enthalpy of ranolazine (−170.02 J/g) also showed a decrease as compared with that of pure drug (−61.40 J/g). The shift and broadness in endothermic peak is probably due to partial reduction in crystallinity. Kinoshita et al correlated this phenomenon to hydrogen bonding between C=O groups of drug and the sylanol group of FLR.
From the FTIR studies the characteristic absorption bands for important functional group of pure drug and drug-loaded microspheres are identified. IR spectra at 1688.2 cm-1(C=O ketone stretch), 1655.8 cm-1 (C=O carboxylic acid stretch), 1437.8cm-1 (C=C stretch aromatic), 1335.8 cm-1 (N-H Stretch), 1295.1 cm-1 (C-N Stretch) and 1257.5 cm-1 stretch). FTIR spectra shown in Figure 5 showed that the characteristics bands of ranolazine were not altered after successful encapsulation without any change in their position, indicating no chemical interactions between the drug and FLR and sodium alginate.
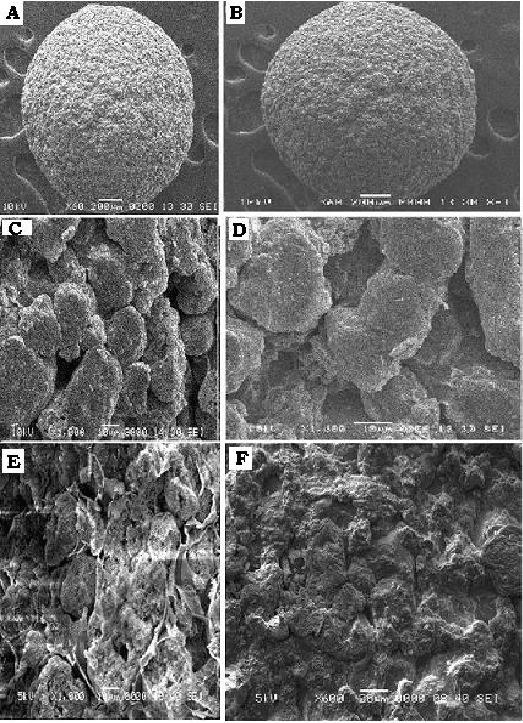
Figure 1: SEM Photograph of beads; A) Blank Placebo Bead B) Drug loaded bead C) and D) Pours FLR E) Drug loaded pours bead (Cross section) F) Incomplete layer of sodium alginate.
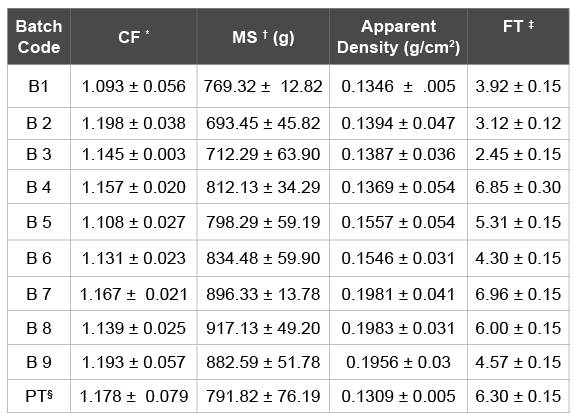
Table 2: Result of different evaluation for beads batches (n=6) * Circulatory factor, † Mechanical strength, ‡ Floating time. § Placebo trial
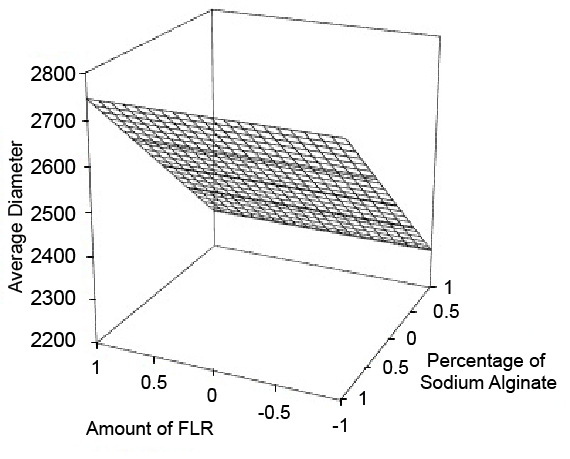
Figure 2: Surface response plot for Average Diameter
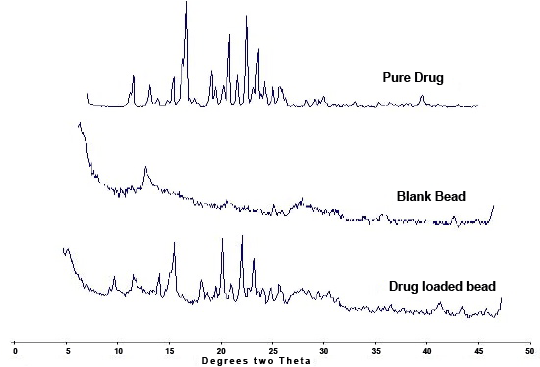
Figure 3: PXRD Analysis of Pure drug (Ranolazine); Blank Beads and drug loaded beads
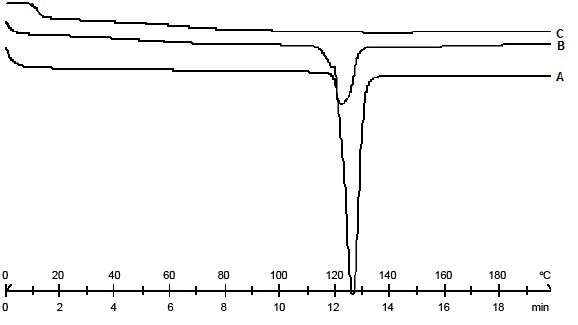
Figure 4: DSC thermograms of A) Pure Ranolazine B) Drug Loaded beads C) Blank Placebo beads
Beads prepared by using 1.0% (w/v) and 1.5% (w/v) sodium alginate solutions showed higher crushing strength ranging from 693 to 917 g probably due to greater bonding of increased strength with increase in concentration of alginate. Higher mechanical strength of beads is important for avoiding breaking and distortion of beads during capsule filling or normal handling especially at the time of large batch production. It is also reflected by the value of coefficient (β2 ), shown in Table 3. The opposite effect of FLR quantity (β1 ) and interaction of it with alginate concentration (β12) on crushing strength of beads may be attributed to increase in bead size with increase in FLR quantity keeping alginate solution concentration constant that probably resulted into weaker and insufficient alginate binding of solid particles (Figure 6). As compared to beads of batch 8 that contained 83.34% FLR out of total powder loaded the beads containing pure FLR showed smaller size and higher mechanical strength. This indicates that size and mechanical strength of beads were not only affected by FLR quantity but also by hydrophobicity of powder loaded in beads. The hydrophobicity of drug probably did not allow stronger bonding between solid particles and alginate that resulted into larger bead size and reduced mechanical strength. The beads of control batch which are devoid of hydrophobic component (drug) probably formed stronger bonds with alginate and hence, produced smaller beads with higher mechanical strength. This is also supported by failure in preparing beads of pure drug.
Buoyancy of beads is directly related to performance of floating-pulsatile drug delivery system since lag time for beads is equivalent to their floating time. Instantaneous in vitro floating behavior was observed for beads of all batches, maybe due to low apparent density provided by porous nature of FLR (Table 2). Floating time was determined as floating parameter; floating time is the time till all of the beads floated on medium. Floating time was primarily controlled by apparent density of beads, which on turn is affected by both quantity of FLR and concentration of sodium alginate solution. Floating time is greater than 6 h for the beads of batches that are containing higher FLR quantity and lower alginate concentration (Table 2). Floating behavior of beads could be explained on the basis of explanation given by Yuasa et al. that polymer forms liquid bridges over pores present on surface of FLR and do not intrude completely into the pores which results into air trapped within granules. Similarly in our study, with the increase in FLR quantity in beads (at same alginate concentration) floating time increases and sinking rate decreases, probably because the number of air trapped pores in beads increases with increase in FLR quantity. Since the control batch does not include ranolazine; a nonporous, denser solid component, therefore control batch beads incorporate higher proportion of FLR compared to drug containing beads that results in lower apparent density of control batch (Figure 7). Results show that with the increase in FLR quantity in beads, keeping alginate concentration constant (1%, w/v), apparent density of beads decreases and floating time increases except for control batch, for which apparent density is lowest but floating time is lesser than floating time. This indicates that hydrophobic component in beads is not only affecting size and mechanical strength but also floating behavior of beads. Hydrophobic nature of ranolazine probably restricts the influx of aqueous medium into the pores of FLR and therefore delays removal of air trapped by aqueous medium.
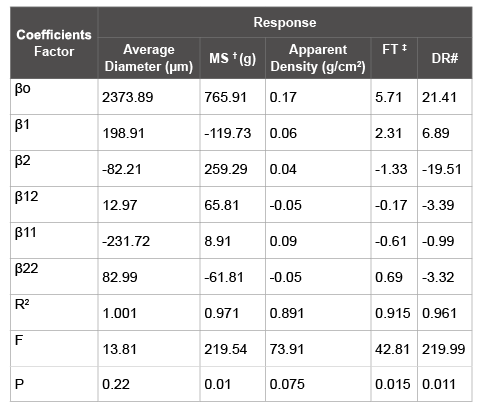
Table 3: Regression Coefficient of different response variables † Mechanical strength, ‡ Floating time. # Drug Release
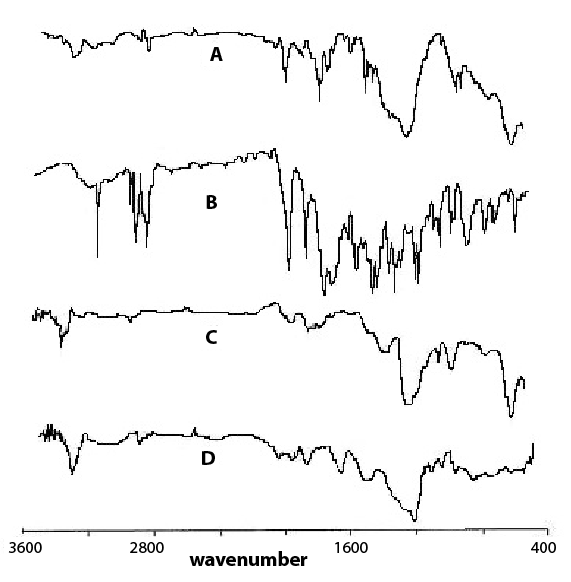
Figure 5: FTIR graphs of A) Drug loaded Beads B) Pure Ranolazine C) FLR D) Sodium alginate
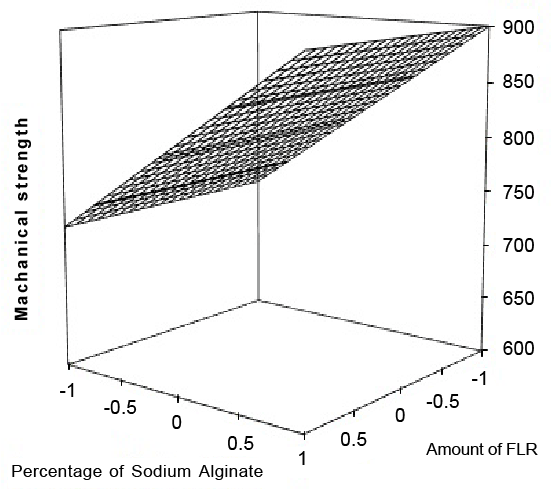
Figure 6: Surface response plot for Mechanical Strength
Comparatively lesser drug release at acidic pH is due to the lack of disintegration and swelling of alginate beads [18]. The drug release was directly related to quantity of FLR and inversely related to concentration of sodium alginate (Table 3).The increase in drug release with increase in FLR quantity and reduction in alginate concentration, may be outcome of two factors; large surface area provided by FLR for drug adsorption and reduction in surface coating of alginate over beads, which thereby increase the effective surface area of surface deposited drug exposed to dissolution medium. In 0.1 N HCl medium drug release from prepared batches over their respective floating time varied from 2.2% to 26.2%. All the batches of beads showed fast disintegration and drug release in SIF probably due to pH dependent swelling of alginate and solubility of ranolazine [19]. Small intestinal transit time for a drug formulation or for a meal is known to be almost constant at about 3 h [19] although the drug release from beads of all batches was quiet rapid, the composition of bead shown significant effect on initial drug release after 3 hrs (Figure 8). This marked effect of alginate concentration on initial drug release 3 hrs may be attributed to formation of stronger and thicker gel formation with increase in concentration of alginate that restrict the drug diffusion through the beads.
A novel type of sustained release multiparticulate floating drug delivery system based on low density porous calcium silicate as drug carrier and pH responsive cross-linked alginate polymer has been developed. Developed formulations showed instantaneous floating with very slow drug release in acidic medium. Quantity of porous carrier and concentration of sodium alginate solution significantly affected the performance of beads. By altering the amount of these two components in formulation floating time of beads could be controlled ranging up to 6.30 h. Drug release from beads in acidic environment was influenced by both quantity of Florite RE® and sodium alginate concentration while initial drug release in simulated intestinal environment was primarily controlled by sodium alginate concentration.
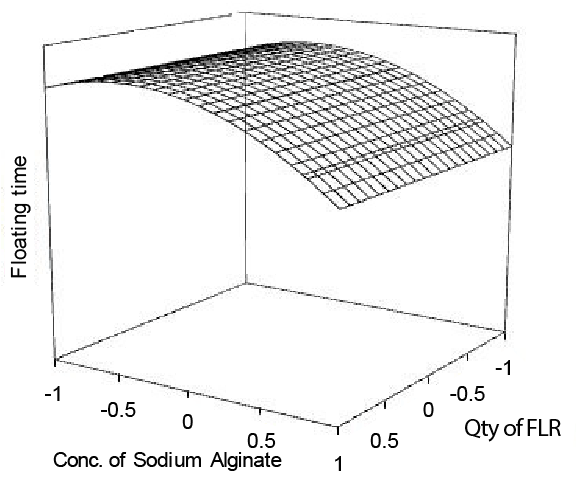
Figure 7: Surface response plots for floating time.
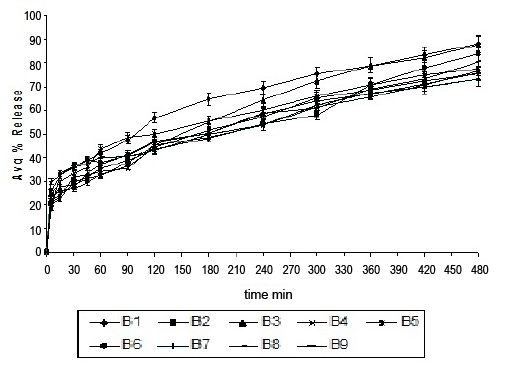
Figure 8: Drug release of different drug loaded calcium alginate beads batches
Author was very much thankful to Mr. Sanjay Mehta and Mr. Samit Mehta for theirencouragement and support during the research.
Download Provisional PDF Here
Article Type: Research Article
Citation: Shinde M, Gunjal P, Gharge V, Gurjar M (2015) Effect of Process Variables on Encapsulated Ranolazine Novel Porous Calcium Silicate. J Drug Res Develop 1(2): doi http://dx.doi.org/10.16966/ 2470-1009.105
Copyright: © 2015 Shinde M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Publication history:
All Sci Forschen Journals are Open Access