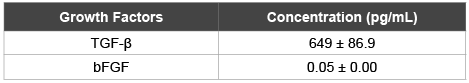
Table 1: Additional growth factors released from hPL-loaded nCS after 48 hours; mean data ± SEM; n=8 samples per group.

Andrew Barone Adam Morrell Rosemary Dziak*
Department of Oral Biology, University at Buffalo School of Dental Medicine, USA*Corresponding author: Rosemary Dziak, Department of Oral Biology, University at Buffalo, State University of New York, Buffalo, NY, USA, 14214; Tel: 716-829-3827; Fax: 716-829- 3942; E-mail: rdziak@buffalo.edu
Introduction: Nanocalcium sulfate represents a novel scaffold with enhanced physical properties. The reported advantages of using nanomaterials over conventional size constructs for fabricating functional scaffold/growth-factor delivery systems are based on their increased surface area for growth factor adsorption and cell attachment. Recent reports on human platelet lysate suggest that it is a reliable source of osteogenic growth factors and is capable of potentiating robust increases in the activity of osteogenic cells To gain more insight on the advantages of fabricating nanomaterials with growth factors, it is important to determine the amount of growth factor content being released and whether or not it is still biologically active. Treating nanomaterials with growth factors may give rise to novel regenerative micro/nano-environments that could be useful for bone tissue engineering applications.
Materials and methods: Scaffolds were fabricated from nanocalcium sulfate mixed with human platelet lysate. The amount of growth factor content released from the scaffold at varying time points was quantified and the biocompatibility of the system was assessed by measuring the resultant activity of mesenchymal stem cells and human osteoblastic cells seeded with the scaffold over a 48 hour incubation period.
Results and conclusion: The incubation of both mesenchymal stem cells and human osteoblastic cells with nano calcium sulfate scaffolds loaded with human platelet lysate induced significant stimulatory effects on the activity of both cell types. This indicates that the growth factor content released from the scaffold system maintained its bioactivity and also provides additional evidence suggesting that nanocalcium sulfate represents an efficient vehicle for the delivery of bioactive molecules capable of enhancing the activity of key cell types involved in osseous formation. These results are supportive of the emerging potential for the development of functional scaffold/growth factor delivery systems that could be used therapeutically in the treatment of craniofacial bone defects.
Nano calcium sulfate; Human platelet lysate; Platelet-derived growth factor; Mesenchymal stem cells; Human osteoblastic cells
nCS: Nano Calcium Sulfate; hPL: Human Platelet Lysate; PDGF: Platelet-derived Growth Factor; Mesenchymal Stem Cells: MSCs; Human Osteoblastic Cells: HOBs
The use of biomaterials for the treatment of osseous defects in humans is a rapidly emerging field of study in bone tissue engineering and dental medicine. One of the most common surgical procedures for bone augmentation and repair is auto genous bone grafting. However, the clinical success and esthetic outcome of this procedure is limited by both the size and severity of the craniofacial bone defect. Auto genous bone grafting is also associated with numerous postoperative complications, including chronic pain, risk of infection, and the potential need for additional grafting surgeries [1]. Furthermore, because patients have only a relatively small volume of graft material available, alternative methods are necessary for the treatment of critical-sized craniofacial bone defects.
Biocompatible scaffold shave received increasing attention as implant/ grafting substitutes in dentistry and represent a promising therapeutic alternative to auto genous bone grafting for the treatment of craniofacial bone defects. An ideal scaffold for bone tissue engineering application should demonstrate high biocompatibility, possess adequate mechanical strength, and exhibit sufficient porosity [2]. Most recently, we have characterized nano-scale calcium sulfate (nCS) as a novel scaffold with enhanced physical properties such as increased surface area for growth factor adsorption and osteoblastic cell attachment [3].
Current research on therapeutic bone regeneration has focused on the development of bio compatible scaffolds that can serve as efficient vehicles for the controlled release of osteogenic growth factors. Platelets are known to contain several growth factors involved in osteogenesis such as platelet derived growth factor (PDGF-BB), transforming growth factor beta (TGF-β), and basic fibroblast growth factor (bFGF) [4]. In this light, the clinical-use of platelet-rich plasma (PRP) for the treatment of osseous defects has been established to offer numerous benefits that include improved bone regeneration with an accompanying decrease in both postoperative pain and risk of infection [5].
However, while many studies on the use of PRP as a therapeutic adjuvant with biomaterials in regenerative medicine have reported encouraging results, its clinical applicability in the treatment of craniofacial bone defects is not well-corroborated [6]. One factor that may contribute to the variability in the reported clinical efficacy of PRP is differing preparation methods. As recently reviewed various techniques for the preparation of PRP are available yet their applications have generated confusion among clinicians because each preparation method leads to a different product (liquid vs. gel) with different biology and potential uses [7-8]. The current, expanding use of human platelet lysate (hPL)-processed from PRP by repeated freeze-thaw cycles-is based upon its reported success at enhancing the activity of mesenchymal stem cells (MSCs ) [9- 11]. Additionally, a recent 2011 study by Dozza and colleagues reported that the incorporation of both MSCs and platelet lysate into a collagen or fibrin scaffold can promote new bone formation around an uncemented hip prosthesis as well as enhance osseo integration at the bone-prosthesis interface [12].
In this study, we chose to focus on the osteogenic potential associated with hPL relative to MSCs as well as human osteoblastic cells (HOBs). Previously, we reported that calcium sulfate (CS) represents an efficient carrier for growth factors that enhance the activity of HOBs in vitro [13]. Given its enhanced physical properties relative to CS, our study aimed to determine whether nCS may serve as a suitable vehicle for the controlled release of growth factors in hPL and also act as a biocompatible scaffold capable of enhancing the activity of MSCs and HOBs.
Human platelet lysate (hPL) was purchased from Zen-Bio Inc (Research Triangle Park, NC). All other reagents were purchased from Sigma-Aldrich (St Louis, MO) unless otherwise stated.
Mesenchymal stem cells (MSCs), prepared from the bone marrow of 6-8 week-old rats according to the protocol approved by the University at Buffalo IACUC previously described in detail (He et al, 2012) were generously provided by Dr. Shuying Yang [14].
Human osteoblastic cells (HOBs) were obtained from primary cultures from human calvariae and were purchased from a commercial supplier, Scien Cell Research Laboratories (San Diego, CA) and cultured according to the supplier’s directions with the exception that the cells were cultured after the second passage in alpha-minimum essential medium (MEM, Gibco, Life Technologies, Grand Island, NY) supplemented with 10% fetal bovine serum (FBS).
We prepared nCS according to the procedure outlined by Park and colleagues [3]. Briefly, a cryo-vacuum method was used to convert dihydrate CS into dehydrate nCS, which was then oven dried to produce hemi hydraten CS. Glow discharge treatment was employed to sterilize the product. We then fabricated hPL-loaded nCS(nCS-hPL) discs by mixing 100-mg ofnCS with 100 µL of hPL. The resultant paste was processed into disc-shaped cylindrical pellets with dimensions of 3-mm and 1-mm thickness in a polyvinylsiloxane (PVS) mold. The discs required an incubation period of approximately 2.5 hours at room temperature in order to harden.
To determine whether nCS is capable of releasing growth factors in hPL in a controlled manner, we measured the amount of PDGF-BB released from nCS-hPL discs incubated in non-supplemented media for 96 hours at 37°C. Given its stimulatory effect on the growth of HOBs, we chose PDGF-BB to serve as a representative growth factor in hPL [15]. For our experiments, nCS-hPL discs were first placed in a 96-well tissue culture plate. We then added 200 µL of media to each well. At four different time points (2, 24, 48, and 96 hours), the media was collected and stored. The discs were then resuspended in 200 µL of fresh media and incubated until the next time point. After 96 hours, the total amount of PDGF-BB present in each sample of collected media was quantified using an ELISA kit (R and D Systems, Minneapolis, MN) and microplate reader.
To determine whether nCS could effectively release growth factors in addition to PDGF-BB, we also measured the amounts of both TGF-β and bFGF released from nCS-hPL discs incubated in non-supplemented media for 48 hours at 37°C. Two separate experiments conducted with each being setup in the same exact manner as our PDGF-BB study just described in detail. However, for these experiments, we collected the media only once, after 48 hours. At this point, the total amount of either TGF-β or bFGF released from nCS-hPL discs was quantified using an ELISA kit (R and D Systems, Minneapolis, MN) and microplate reader.
The tetrazolium salt (MTT) assay was used to assess the biocompatibility of nCS-hPL relative to two different cell types: MSCs and HOBs. In our study, nCS-hPL discs were first placed in a 96-well tissue culture plate. Then 200µL of cells suspended in FBS-supplemented media (10% v/v) was added to each well. Three separate experiments were conducted. For the first, MSCs were seeded with nCS discs loaded either with hPL, diluted hPL (50% v/v), or without hPL. For the second, MSCs were seeded either without nCS discs in media or with nCS discs in hPL-supplemented media (10% v/v). For the third, HOBs were seeded either with nCS-hPL discs or nCS discs in media. In each of the experiments, nCS discs not loaded with hPL were instead mixed with non-supplemented media at a ratio of 100-mg nCS/ 100µL MEM and served as control groups. After a 48 hour incubation period at 37°C, MTT reagent was added to the cells for 2 hours and the assay was conducted as previously described in detail [16].
The growth factor release experiment revealed that nCS can release PDGF-BB in a controlled manner. After a small burst following the initial 2 hour incubation period, the release rate of PDGF-BB gradually decreased at each subsequent time point (Figure 1). At 96 hours, approximately 15% (340 ± 40 pg mL-1) of the total PDGF-BB content (≈2,500 ± 10 pg mL-1) in each nCS-hPL disc was released into the media. In addition to PDGF-BB, nCS-hPL discs were also shown to release both TGF-β and bFGF, thereby providing further indication that nCS represents an efficient vehicle for the release of bioactive molecules (Table 1).

Table 1: Additional growth factors released from hPL-loaded nCS after 48 hours; mean data ± SEM; n=8 samples per group.
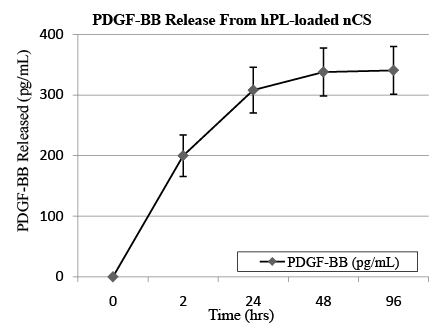
Figure 1: Cumulative PDGF-BB release profile from hPL-loaded nCS; mean data ± SEM; n=8 samples.
The biocompatibility of nCS-hPL discs was assessed by measuring the resultant activity displayed by MSCs seeded with the discs after 48 hours. Two experiments with MSCs were conducted. In the first experiment, the activity of MCSs seeded with nCS-hPL discs was compared to the activity of MSCs seeded with nCS discs and MSCs seeded with nCS discs loaded with a diluted solution of hPL (nCS-50% hPL). The group of MSCs seeded with nCS-hPL discs exhibited significantly greater activity than each of the other treatment groups (Figure 2). The second experiment was performed in order to control for the potential influence of nCS itself on MSC activity. When the activity of MSCs seeded without nCS discs was measured, no significant difference was observed relative to the activity of MSCs seeded with nCS discs in media lacking hPL (Figure 3). However, when MSCs were seeded with nCS discs and incubated in hPLsupplemented media, they exhibited significantly greater activity than each of the other treatment groups.
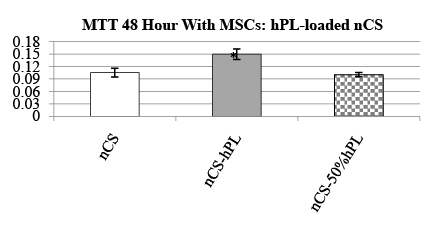
Figure 2: MTT cell activity of MSCs seeded with hPL-loaded nCS discs;mean data ± SEM; n=3-4 samples per group; * = significantly different from other treatment groups; = p<0.05 ANOVA.
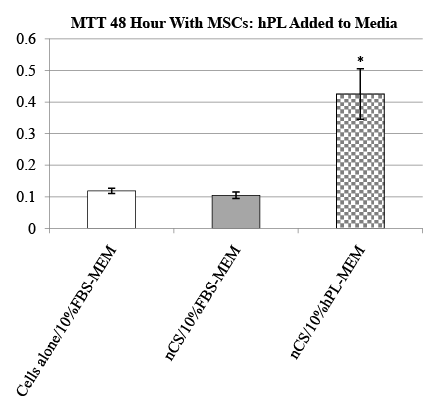
Figure 3: MTT cell activity of MSCs seeded with nCS discs inhPLsupplemented media;mean data ± SEM; n=4 samples per group; * = significantly different from other treatment groups; = p<0.05 ANOVA.
The biocompatibility of nCS-hPL discs was also assessed by measuring the resultant activity displayed by HOBs seeded with the discs after 48 hours. In the experiment, the activity of HOBs seeded with nCS-hPL discs was compared to the activity of HOBs seeded with nCS discs. The group of HOBs seeded with nCS-hPL discs exhibited significantly greater activity than the other treatment group (Figure 4).
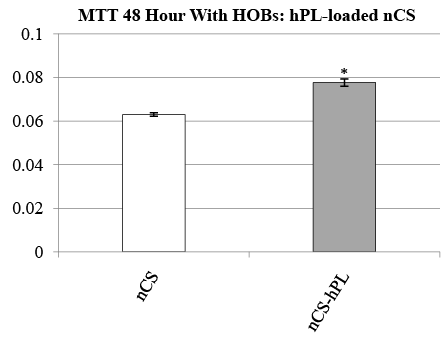
Figure 4: MTT cell activity of HOBs seeded with hPL-loaded nCSdiscs; mean data ± SEM; n=8 samples per group; * = significantly different from cells seeded with nCS discs; = p<0.05 ANOVA.
In this study, we have shown that nCS-hPL discs represent biocompatible scaffolds that permit the release of growth factors in a controlled manner and offer an attractive strategy for improving bone regeneration through enhancing the activity of MSCs and HOBs. In a prior study, we demonstrated that when nCS and CS discs are fabricated with equal amounts of aqueous recombinant human PDGF-BB (rhPDGFBB), nCS releases more rhPDGF-BB than CS over a 20 day period in vitro [3]. Clinically, the use of rhPDGF-BB mixed with bone allograft has been shown to promote robust osseous regeneration in periodontal bony defects [17]. However, due to costs and regulatory issues as well as the inherent complexity of the natural physicochemical processes underlying osseous regeneration, the clinical-use of human recombinant growth factors is becoming increasingly prohibitive [18]. In this light, PRP represents a more practical source of growth factors for therapeutic use in dentistrycompared to recombinant proteins-because it is an autologous preparation and thereby eliminates concerns about immunogenic reactions or disease transmission [19].
However, as recently reviewed the current dental literature regarding the benefits ofPRP therapy is inconclusive and human trials show conflicting results [6]. For instance, in a 1998 study by Marx and coworkers involving patients with mandibular continuity defects, patients treated with bone grafts mixed with PRP showed faster and improved bone regeneration compared to patients treated with bone grafts alone [20]. Alternatively, in a 2005 study by Thor and coworkers involving subjects requiring alveolar ridge augmentation to enable the placement and integration of titanium implants, no obvious positive effects of PRP on implant survival rate and bone graft healing could be demonstrated [21].
With regard to functionalizing biomaterials with osteogenic growth factors, in a 2013 study by Leotot et al. involving mice with osseous defects, mice treated with hPL-coated HA/β-TCP scaffolds demonstrated improved and more dense bone formation compared to mice treated with HA/β-TCP scaffolds alone [22]. Our study, in turn, provides further indication of the potential for the development of functional scaffold/ growth-factor delivery systems. Furthermore, compared to HA/β-TCP, nCS is nanostructured and has enhanced physical characteristics such as increased surface area and porosity. Thus, in fabricating nCS discs with hPL, osteogenic growth factors become disseminated throughout the architecture of the scaffold system, which protects them from degradation and helps to keep their local levels constant.
The potential for nCS loaded with hPL to serve as a functional scaffold/ growth factor delivery system was established. PDGF-BB, TGF-β, and bFGF were released from the scaffold and had sufficient bioactivity to either directly or indirectly enhance the activity of MSCs as well as HOBs. This study expands on the evidence that nCS represents an efficient vehicle for the controlled released of bioactive molecules. Moreover, our results suggest that nCS-hPL scaffolds give rise to novel regenerative micro/ nano-environments that could be useful for bone tissue engineering applications.
Download Provisional PDF Here
Article Type: Research Article
Citation: Barone A, Morrell A, Dziak R (2016) Fabrication and Characterization of NanoCalcium Sulfate and Human Platelet Lysate as a Growth Factor Delivery System. Int J Dent Oral Health 2(4): http://dx.doi.org/10.16966/2378-7090.172
Copyright: © 2016 Dziak R, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Publication history:
All Sci Forschen Journals are Open Access