
Full Text
Association of Periodontitis and Systemic Diseases
Muhammad Wasif Haq1, Farzeen Tanwir2*, Saba Tabassum3, Madiha Nawaz4, Mohammad Faraz Siddiqui51Student of Masters of Speech Pathology, Flinders University, Adelaide, Australia
2*Associate Professor and Head of Periodontology Department, Ziauddin University, Karachi, Pakistan
3House Officer in Hamdard College of Medicine & Dentistry, Karachi, Pakistan
4Private Medical Practice, Islamabad, Pakistan
5Student of Masters in Epidemiology and Biostatistics, Aga Khan University, Karachi, Pakistan
*Corresponding author: Farzeen Tanwir, Director of Post graduate Studies and Research Associate Professor, and Head, Department of Periodontology, Ziauddin University, Pakistan; E-mail: farzeen_tanwir@yahoo.com
Abstract
Aim: To determine the frequency of periodontitis in patients with systemic diseases admitted in the hospital.
Method: This cross sectional study consists of a total of 450 patients. The data was obtained from Pakistan Institute of Medical Sciences, Capital Development Authority, Islamic International Dental College, Islamabad and Jinnah Postgraduate Medical Center, Karachi. The patients in the study were suffering from diseases such as diabetes mellitus, cardiovascular disorders (with and without diabetes mellitus), respiratory disorders, gastrointestinal disorders, hepatic disorders, renal disorders, bone/ joint disorders and hypertension. Periodontal status was examined with the help of sterilized dental mirror, periodontal probe, wooden tongue depressor and torch.
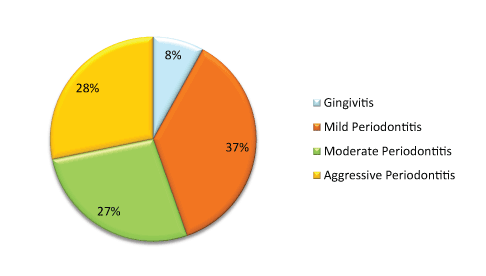
Results: Systemic diseases negatively impacting periodontal status are listed in the descending order of severity: Diabetes mellitus+cardiovascular disease> Diabetes mellitus> Cardiovascular disease> Bone/joint disorders> Renal p roblems> Hypertension> Hepatic disease> Gastrointestinal disorders> Respiratory problems. In terms of periodontitis, 37% of population experienced mild periodontitis, 28% had aggressive periodontitis, 27% had moderate periodontitis while 8% had gingivitis.
Conclusion: The results of the study indicate the association between systemic illnesses and development of severe periodontitis. This implies that improvement of oral health can lead to betterment of the systemic health which will lead to better quality of life for the patient. Therefore we recommend collaboration between dentists and medical physicians through which focus on prevention and treatment of oral as well as systemic health can be achieved.
Keywords
Periodontitis, Systemic health, Oral health
Introduction
Oral cavity comprises of over 700 bacterial species residing as normal oral flora along with other fungal species [1]. In periods of lowered immunity, these organisms can not only cause the development of local opportunistic infections but may also initiate or aggravate the systemic illnesses. Because of the anatomical connection of mouth with respiratory and digestive tract in addition to its’ rich vascular supply ensures a potential source of spread of infectious agents from the mouth to the body. Furthermore, mouth serves as a passage for the entry of various pathogens through ingestion of food substances. For these reasons oral hygiene is said to be an indicator of systemic health as many of the infections and their manifestations appear initially in the mouth than elsewhere in the body [2]. However, oral care is often neglected as many consider oral hygiene to be less important than systemic health and patients visit medical practitioner more frequently than dentist [3]. This negligence on the patient’s part coupled with lack of awareness and training of general medical practitioners to examine oral cavity can lead to further deterioration of oral hygiene as well and chances of finding if any systemic disease has arisen secondarily to poor oral health are missed [4].
A neglected oral hygiene can lead to excessive accumulation of plaque and calculus which can eventually result in the most common infections such as caries, gingivitis and periodontitis [5]. Periodontitis is one of the most common chronic infections occurring in the body [6]. Studies on the prevalence of periodontitis reveal as high as 90% prevalence with gingivitis affecting almost everyone [7,8]. Earlier studies conducted on Pakistani school children showed a 100% prevalence of the periodontitis [9]. The disease significantly adds to the global burden of disease [10]. The consequences and implications of periodontitis are not only limited to the oral cavity but are far reaching. Compromised oral hygiene has been linked to an increased risk of metabolic disorders such as diabetes mellitus, cardiovascular diseases and diseases of respiratory tract, gastrointestinal tract, renal, hepatic and bone/joints [11]. Porphyromonas gingivalis; a periodontal pathogen can increase the risk for diabetes mellitus, cardiovascular disorders, preterm birth, non alcoholic liver disease as well as autoimmune antibodies formation in Rheumatoid arthritis patients [12,13]. On the other hand, severe periodontal infection can cause systemic inflammation thereby implying a bidirectional relationship between deficient oral hygiene and systemic illnesses which means that negligence of one can aggravate the disease in the other [14]. Recently it has also been shown that patients suffering from periodontitis have higher number of β-lactamase producing microorganisms, therefore, improvement of oral hygiene can lead to better response of the body to therapy and medications thereby improving overall health of the body [15].
The aim of this study, which is a part of research project on association of periodontitis with systemic illnesses, was to evaluate the association of periodontitis in patients suffering from various systemic illnesses admitted in the hospitals. To the authors’ knowledge, there has been insufficient data recorded in Pakistan about association of periodontitis in patients with different systemic diseases.
Materials and Methods
The ethical approval for this observational study was obtained from Ethics committee at Ziauddin University, Karachi, Pakistan. The sample size was determined by online sample size calculator by Raosoft. The resulting recommended sample size was 377, which were increased to 520 for more accuracy. Data collection was done between the months of July-November 2012 from two public sector; tertiary care hospitals in Islamabad namely Pakistan Institute of Medical Sciences (P.I.M.S.), Capital Development Authority (C.D.A.) hospital, outpatient department at Islamic International Dental College and Hospital, Islamabad and Jinnah Postgraduate Medical Center (J.P.M.C.) Karachi, which is also a public sector, tertiary care hospital. Patients from nine systemic illnesses were chosen for this study which is listed in (Table 1).
Conscious patients having permanent dentition and suffering from one disease were selected for the study after taking informed consent. Patients with acute infections, pain, pregnancy, loss of teeth due to trauma or any accident and total absence of teeth were excluded which resulted in total sample size of 450 patients. For every disease; 50 patients were chosen. The patients were asked about their disease and the reason for visiting hospital. Their medical reports were assessed to rule out co-morbidities.
Dental examination was performed using sterilized dental mirror, periodontal probe, wooden tongue depressor and a torch light. Data for systemic disease and periodontal status was recorded for every patient. The normal number of teeth was taken to be 28 excluding 3rd molars for this study.
| Disease | Conditions Present |
|---|---|
| Diabetes Mellitus (D.M.) | Type 1 & Type 2 |
| Cardiovascular disorders (C.V.S.) | Myocardial infarction, Angina Pectoris |
| Respiratory disorders | Tuberculosis, Asthma, Chronic obstructive pulmonary disease (C.O.P.D.), Pneumonia |
| Gastrointestinal disorders (G.I.) | Gastric ulcer, Gastritis, Inflammatory bowel disease, Typhoid. |
| Hepatic disorders | Hepatitis B & C, Hepatocellular carcinoma. |
| Renal disorders | Chronic kidney failure, proteinuria. |
| Bone/joint disorders | Rheumatoid arthritis, osteoarthritis, osteoporosis. |
| Hypertension (H.T.N.) | Primary hypertension |
| Diabetes Mellitus + Cardiovascular patients (D.M.+ C.V.S.) | Diabetic patients with co-morbid heart disease. |
Table 1: Systemic diseases chosen for the study along with the most frequent forms of the disorders seen in the sample.
According to Periodontal disease classification, gingivitis was taken as inflammation of gums. Periodontitis (generalized with > 30 % sites covered) is categorized as mild, moderate and aggressive, with mild having 1-2 mm of CAL and moderate being 3-4mm and aggressive being >5 mm of CAL (clinical attachment loss). (AAP: Annals of Periodontology- Vol 4.Classification).
For statistical analysis, Statistical Package for Social Sciences (S.P.S.S.) version 16 was used to calculate the mean for each variable along with the standard error. Mean age of the sample was calculated. The mean for periodontal diseases for each systemic disease along with standard deviation was estimated. Kruskal Wallis test was used to find degree of statistically significant association between systemic diseases and periodontal diagnosis, followed by Mann–Whitney U test to determine p value between diseases. The level of significance was set at p< 0.05 (95% confidence interval).
Results
There were equal number of males and females in the selected sample of 450 patients with the mean age of 43.28 years (± 14.45) and the age range as 13-84 years. 92% of the sample has periodontitis while 8% had gingivitis (Graph 1).

Periodontally majority of the patients had mild form of periodontitis while 27% and 27% had moderate and severe periodontitis respectively. Diabetic patients with or without associated C.V.S. problems had highest score of periodontal problems which did not differ from scores obtained for C.V.S. and bone/joint diseases patients. In both the categories of D.M.; with and without C.V.S. problems, the maximum number of patients had aggressive periodontitis (Graph 2). D.M. appears to be the only disease in the current study in which none of the patient had gingivitis suggesting the prevalence of periodontitis as high as 100% in diabetics included.
Cardiovascular patients had relatively better oral hygiene compared to D.M. patients, though the difference was non significant (Graph 2, Table 3). The C.V.S. patients in the present study had moderate to aggressive periodontitis (Graph 2). There were no significant differences between C.V.S. patients and patients of D.M., D.M.+C.V.S., renal, bone/joint problems and H.T.N. suggesting a similar pattern of oral health as that of C.V.S. patients amongst all these.
Disease |
D.M |
C.V.S |
Respiratory |
G.I |
Hepatic |
Renal |
Bone/ Joint |
H.T.N |
D.M+ CVS |
P value |
Diagnosis |
3.2 |
3.1 |
2.14 |
2.2 |
2.38 |
2.84 |
2.96 |
2.8 |
3.2 |
0.00 |
Table 2: Periodontal score of patients suffering from various systemic diseases along with the p value and SD. 1= Gingivitis, 2= Mild periodontitis, 3= Moderate periodontitis, 4=Aggressive periodontitis.
Disease |
C.V.S |
Respiratory |
G.I |
Hepatic |
Renal |
Bone & Joint |
H.T.N |
D.M+CVS |
D.M |
0.51 |
0.00 |
0.00 |
0.00 |
0.05 |
0.18 |
0.02 |
0.62 |
C.V.S |
- |
0.00 |
0.00 |
0.00 |
0.20 |
0.49 |
0.11 |
0.27 |
Respiratory |
|
- |
0.88 |
0.12 |
0.00 |
0.00 |
0.00 |
0.00 |
G.I |
|
|
- |
0.23 |
0.00 |
0.00 |
0.00 |
0.00 |
Hepatic |
|
|
|
- |
0.01 |
0.00 |
0.02 |
0.00 |
Renal |
|
|
|
|
- |
0.55 |
0.78 |
0.03 |
Bone & Joint |
|
|
|
|
|
- |
0.38 |
0.11 |
H.T.N |
|
|
|
|
|
|
- |
0.02 |
Table 3: Comparison of oral health between patients of systemic diseases
The sample with bone/joint diseases such as osteoporosis, osteopenia and arthritis; osteoarthritis and rheumatoid arthritis also had higher prevalence of periodontitis in the study (Table 2).
After D.M., bone/joint disease is the only disease with least number of patients suffering from minimal level of periodontal disease (Graph 2).
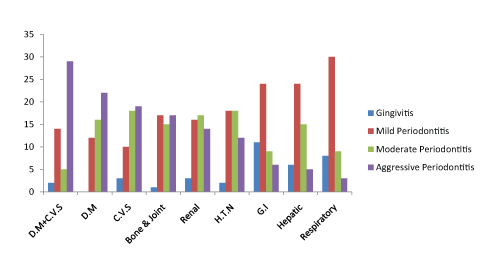
Graph 2: Periodontal status of patients with systemic diseases represented individually.
Maximum number of renal patients had moderate periodontitis (Graph 2). H.T.N. patients had similar periodontal health as renal patients with equal number of patients with mild and moderate periodontitis (Graph 2).
In this study, majority of hepatic patients had mild periodontitis with only 5 patients suffering from aggressive periodontitis.
Except for respiratory and G.I. diseases patients; there were significant differences with all other diseases (Table 3).
Patients with respiratory disorders had least effect on periodontal health as few patients suffered aggressive periodontitis (Graph 2). Most of the G.I. patients suffered from mild periodontitis and this disease has a maximum count in gingivitis (Table 3).
The results from the current study show a relatively weak association between periodontal health and G.I. disorders (Table 3, Graph 2). The comparisons between oral healths in patients with various systemic diseases along with the p-value are shown in Table 3. Periodontal status for each disease category is represented individually in Graph 2.
Discussion
There is highly significant association between periodontal and systemic diseases (p=0.00). It can be seen that systemic diseases always affect oral hygiene negatively as none of the patient had sound oral health (Graph 1). Periodontally majority of the patients had mild form of periodontitis while 27% and 27% had moderate and severe periodontitis respectively.
Diabetic patients with or without associated C.V.S. problems had highest score of periodontal problems which did not differ from scores obtained for C.V.S. and bone/joint diseases patients. The non significant association between D.M. and D.M. + C.V.S., C.V.S. patients and bone/ joint patients imply that periodontal health of patients suffering from these systemic diseases is alike (Table 3). Both types of D.M. are known risk factor for periodontitis and periodontitis is cited as the 6th most common complication of D.M. [16,17]. The poor blood sugar control puts diabetic patients at 2.8 times higher risk of periodontitis and almost 4.2 times at risk of alveolar bone loss relating hyperglycemia with increased chances of periodontitis [18]. On the other hand, improvement in oral hygiene is associated with a better control of blood sugar and a subsequent decline in the diabetes related complications such as retinopathy, neuropathy, proteinuria, C.V.S. complications and ketoacidosis [16,18]. Severe periodontal disease can increase mortality by diabetic nephropathy 8.5 times compared to people with mild to moderate periodontal disease [19]. In D.M. patients, periodontitis is a predictor of mortality from ischemic heart disease. Studies have shown higher periodontal inflammation in diabetic patients and poor glycemic control is in turn linked with periodontal attachment loss leading to tooth loss [19,20]. In both the categories of D.M.; with and without C.V.S. problems, the maximum number of patients had aggressive periodontitis (Graph 2). D.M. appears to be the only disease in the current study in which none of the patient had gingivitis suggesting the prevalence of periodontitis as high as 100% in diabetics included. The oral health of diabetic individuals should be given special care as periodontitis can also increase the resistance to insulin [21]. This may cause increased risk for D.M. in periodontitis patients as well can cause exacerbation of diabetes in diabetic patients [22].
Cardiovascular patients had relatively better oral hygiene compared to D.M. patients, though the difference was non significant (Graph 2, Table 3). Numerous studies have found association between C.V.S. and periodontal disease with one study suggesting 25-90% increased risk of C.V.S. disorders in patients with severe periodontitis [20]. Another study showed that 91% of cardiovascular patients have moderate to severe periodontitis [23]. Poor oral hygiene can produce bacteremia when patient chews food or brushes teeth which can lead to increased inflammatory cytokine production in the body, the level of bacteremia being proportional with the degree of inflammation and infection in the oral cavity [24,20]. Therefore, it is suggested that patients at risk of infective endocarditis and cardiovasculat disorders should maintain best possible oral hygiene. Not only C.V.S. disease is a risk factor for periodontitis, the latter also increases the risk of thrombus and atherosclerosis as people who suffered from C.V.S. ischemia; non hemorrhagic stroke have always some form of periodontitis [25-27]. Oral bacteria such as Streptococcus sanguis and Porphyromonas gingivalis induce platelet aggregation and promote thrombus formation [28]. Likewise; periodontal pathogens have also been identified in atherosclerotic lesions listing periodontitis as a risk factor for C.V.S. disease [29]. The C.V.S. patients in the present study had moderate to aggressive periodontitis (Graph 2). There were no significant differences between C.V.S. patients and patients of D.M., D.M.+C.V.S., renal, bone/ joint problems and H.T.N. suggesting a similar pattern of oral health as that of C.V.S. patients amongst all these.
The sample with bone/joint diseases such as osteoporosis, osteopenia and arthritis; osteoarthritis and rheumatoid arthritis also had higher prevalence of periodontitis in the study (Table 2). After D.M., bone/joint disease is the only disease with least number of patients suffering from minimal level of periodontal disease (Graph 2). The bone loss is a common characteristic between periodontitis and osteoporosis due to which a possible association between the two is often rejected [30]. Many studies have presented significant association between estrogen deficiency and periodontitis [31,32]. It has been seen that in periodontitis; osteoblasts cells decrease in number while osteoclastic activity is stimulated which can increase the chances of developing osteoporosis in periodontitis patients [33,34]. The degree of periodontal inflammation is also higher in postmenopausal women affected with osteopenia and osteoporosis [35,36]. A decline in alkaline phosphatase is associated with periodontal attachment loss [37]. The degree of periodontitis is directly linked with severity of rheumatoid arthritis and improving oral health leads to reduction in severity of rheumatoid arthritis [38,39]. In the current study, the patients of respiratory, G.I. and hepatic disorders were significantly different from bone/joint diseases patients.
Maximum number of renal patients had moderate periodontitis (Graph 2). Periodontally; significant findings were seen with D.M., D.M.+C.V.S., hepatic, G.I. and respiratory patients. It has been reported that predialysis and hemodialysis patients have severe periodontitis [40]. Similarly causative link between glomerulonephritis and periodontitis has also been observed [41]. Patients with hyperoxaluria and uremia have poor oral hygiene, greater alveolar bone loss which causes tooth loss as well [42,43]. Regarding tooth loss; nephropathy is a risk factor for partial tooth loss and edentulous patients are found to be commonly suffering from chronic kidney disease [44,45]. Chronic renal failure increases plaque level which if persists can lead to periodontal problems [5,46]. However, some studies have observed no significant results linking kidney disorders with periodontitis [47,48].
H.T.N. patients had similar periodontal health as renal patients with equal number of patients with mild and moderate periodontitis (Graph 2). The poor oral health is linked to elevation of blood pressure in H.T.N. patients and consequently causes poor blood pressure control in the older patients [49,50]. There have been refuting reports as well which do not establish any link between the two [51]. H.T.N. favors the development of periodontitis and severe periodontitis in turn is associated with elevation of blood pressure which can increase the left ventricular mass [52-54]. The disease differed significantly with D.M., D.M.+C.V.S., hepatic, G.I. and respiratory disorders.
Regarding the possible association between periodontitis and liver diseases; C-reactive protein increases in patients with periodontitis [55]. This increased synthesis can be due to local production of the inflammatory protein by the diseased gingival tissue or by the liver enhancing thereby the risk of C.V.S. disorder due to atheroma formation [27,28,56]. This implies that some systemic diseases may raise the chance of other systemic diseases which in turn are also linked with periodontitis. In males affected with periodontitis, an increase in alanine aminotransferase was observed, however authors found no such rise in the enzyme in female population [57]. In a study, Hepatitis C patients were reported to have poor oral hygiene [58]. Several periodontal bacteria have been isolated in patients with pyogenic liver abscess and improving the oral hygiene reduces liver injury and mortality in chronic liver disease patients [59-61]. In this study, majority of hepatic patients had mild periodontitis with only 5 patients suffering from aggressive periodontitis. Except for respiratory and G.I. diseases patients; there were significant differences with all other diseases (Table 3).
Patients with respiratory disorders had least effect on periodontal health as few patients suffered aggressive periodontitis (Graph 2). The literature states that oral bacteria can be aspirated to cause pulmonary infections and dental plaque can initiate as well as aggravate pneumonia [62,63]. Chlamydophila pneumonia; a pathogen involved in pneumonia is shown to increase chances for periodontitis as well as atherosclerosis [64,65]. There has been discussion about poor periodontal health in asthmatic individuals and the decrease in bone density especially in mandible in patients using inhaled corticosteroids [66]. Periodontal attachment loss can also cause a decrease in lung function [67]. Maintaining good oral hygiene in C.O.P.D. patients decreases the frequency of exacerbation in chronic periodontitis patients which relates periodontitis as a risk factor for C.O.P.D [68,69]. In C.O.P.D. patients; chronic gingivitis is a common finding [70]. In the current study; majority of the patients remained on lower end of periodontal diseases (Graph 2) with only 3 patients having aggressive periodontitis. The disease was non-significantly related to G.I. and hepatic disorders only.
Most of the G.I. patients suffered from mild periodontitis and this disease has a maximum count in gingivitis (Table 3). Oral bacteria are related to G.I. diseases and likewise. Helicobacter pylori is said to exist in greater number in oral cavities of gastric ulcer patients and it can cause poor oral health [71,72]. This bacterium is said to produce Hydrogen sulfide which causes oral malodour [73]. Recent evidence has linked Hydrogen sulfide with increased risk of alveolar bone resorption due to increased osteoclastic cell activation [74]. As alveolar bone resorption is a characteristic feature of periodontitis; H. pylori is found increasingly in oral cavities of patients suffering from periodontitis [75]. There have also been some reports associating the bacteria with Insulin resistance which can increase the chances of D.M [76]. Prevalence of periodontitis in inflammatory bowel disease, Crohn’s disease and ulcerative colitis is also high suggesting that G.I. disorders impact oral hygiene negatively [77,78]. The results from the current study show a relatively weak association between periodontal health and G.I. disorders (Table 3, Graph 2). The non-significant association was observed with hepatic and respiratory patients only in this study.
The strength and weakness of the study deserve mention. This is the first study to assess the severity of periodontitis in patients with multiple systemic illnesses. The sample size was increased to have better evaluation of periodontal health status. The weaknesses of the study include failure to include lab tests of different biomarkers of systemic and periodontal disease, which will be considered for our ongoing project. Another limitation is healthy subjects will be added and compared with the patients suffering from various diseases, which is necessary to know the baseline prevalence of Periodontitis among the people of Pakistan.
The periodontal health of patients experiencing systemic illnesses was noted to be extremely worse. The bidirectional relationship between periodontal diseases and systemic illnesses imply that improving oral health can lead to betterment of systemic health. Therefore it is the need of the hour to form collaborative units between dentists, general physicians and specialists to focus not only on systemic health but also on oral health. Many of these diseases can be prevented and improved by improving oral hygiene; therefore, it is the need of the hour.
We would like to pay special thanks to Dr. Haq Nawaz, Dr. Usman Nawaz, Dr. Asad Noor at C.D.A. hospital, Dr. Rizwan at P.I.M.S. hospital and to Arsalan Yahya for the valuable help offered during this study.
References
- Ahn J, Chen CY, Hayes RB (2012) Oral microbiome and oral and gastrointestinal cancer risk. Cancer Causes Control, 23: 399-404.[Ref.]
- Percy MS (2008) Oral health of adolescents--it's more than dental caries. MCN Am J Matern Child Nurs, 3: 26-31. [Ref.]
- Bell GW, Smith GL, Rodgers JM, Flynn RW, Malone CH (2008) Patient choice of primary care practitioner for orofacial symptoms. Br Dent J. 204: 669-673. [Ref.]
- Hein C, Schönwetter DJ, Iacopino AM (2011) Inclusion of oral-systemic health in predoctoral/undergraduate curricula of pharmacy, nursing, and medical schools around the world: a preliminary study. J Dent Educ 75: 1187-1199. [Ref.]
- Haq MW, Batool M, Ahsan SH, Sharma G (2011) Efficacy of antiplaque mouthwashes: a five-day clinical trial. Gen Dent 59: e110-115. [Ref.]
- Seymour GJ, Ford PJ, Cullinan MP, Leishman S, Yamazaki K (2007) Relationship between periodontal infections and systemic disease. Clin Microbiol Infect 4: 3-10. [Ref.]
- Pihlstrom BL, Michalowicz BS, Johnson NW (2005) Periodontal diseases. Lancet 366:1809-1820.[Ref.]
- Huck O, Tenenbaum H, Davideau JL (2011) Relationship between Periodontal Diseases and Preterm Birth: Recent Epidemiological and Biological Data. J Pregnancy 2011: 164654. [Ref.]
- Day CD, Shourie KL (2011) The incidence of periodontal disease in the Punjab. Ind.Med.Res 1944:32:47-51.
- Jin LJ, Armitage GC, Klinge B, Lang NP, Tonetti M et al. (2011) Global oral health inequalities: task group--periodontal disease. Adv Dent Res. 23: 221-226. [Ref.]
- Renvert S (2003) Destructive periodontal disease in relation to diabetes mellitus, cardiovascular diseases, osteoporosis and respiratory diseases. Oral Health Prev Dent 1: 341-357. [Ref.]
- Yoneda M, Naka S, Nakano K, Wada K, Endo H, et al. (2012) Involvement of a periodontal pathogen, Porphyromonas gingivalis on the pathogenesis of non-alcoholic fatty liver disease. BMC Gastroenterol, 12: 16. [Ref.]
- GR Persson (2012) Rheumatoid arthritis and periodontitis – inflammatory and infectious connections. Review of the literature. J Oral Microbiol 4:10.3402. [Ref.]
- Nibali L, D'Aiuto F, Griffiths G, Patel K, Suvan J, et al. (2007) Severe periodontitis is associated with systemic inflammation and a dysmetabolic status: a case-control study. J Clin Periodontol.34: 931-937. [Ref.]
- Rams TE, Degener JE, van Winkelhoff AJ (2012) Prevalence of β-lactamase-producing bacteria in human periodontitis. J Periodontal Res 48: 493-499. [Ref.]
- Watanabe K (2011) Periodontitis in diabetics: is collaboration between physicians and dentists needed? Dis Mon 57: 206-213. [Ref.]
- Löe H (1993) Periodontal disease. The sixth complication of diabetes mellitus. Diabetes Care 16: 329-334. [Ref.]
- Weidlich P, Cimões R, Pannuti CM, Oppermann RV (2008) Association between periodontal diseases and systemic diseases. Braz Oral Res 1: 32-43. [Ref.]
- Preshaw PM, Alba AL, Herrera D, Jepsen S, Konstantinidis A, et al. (2012) Periodontitis and diabetes: a two-way relationship. Diabetologia 55: 21-31. [Ref.]
- Kim J, Amar S (2006) Periodontal disease and systemic conditions: a bidirectional relationship. Odontology 94: 10-21. [Ref.]
- Lalla E, Papapanou PN (2011) Diabetes mellitus and periodontitis: a tale of two common interrelated diseases. Nat Rev Endocrinol 7: 738-748. [Ref.]
- Colombo NH, Shirakashi DJ, Chiba FY, Coutinho MS, Ervolino E, et al. (2012) Periodontal disease decreases insulin sensitivity and insulin signaling. J Periodontol 83: 864-870. [Ref.]
- Geerts SO, Legrand V, Charpentier J, Albert A, Rompen EH (2004) Further evidence of the association between periodontal conditions and coronary artery disease. J Periodontol 75: 1274-1280. [Ref.]
- Lockhart PB, Brennan MT, Thornhill M, Michalowicz BS, Noll J, et al. (2009) Poor oral hygiene as a risk factor for infective endocarditis-related bacteremia. J Am Dent Assoc 140: 1238-1244. [Ref.]
- D'Aiuto F, Parkar M, Andreou G, Brett PM, Ready D, et al. (2004) Periodontitis and atherogenesis: causal association or simple coincidence? J Clin Periodontol 31: 402-411. [Ref.]
- Haraszthy VI, Zambon JJ, Trevisan M, Zeid M, Genco RJ (2000) Identification of periodontal pathogens in atheromatous plaques. J Periodontol 71: 1554-1560. [Ref.]
- Wu T, Trevisan M, Genco RJ, Dorn JP, Falkner KL, et al. (2000) Periodontal disease and risk of cerebrovascular disease: the first national health and nutrition examination survey and its follow-up study. Arch Intern Med160 : 2749-2755. [Ref.]
- Li X, Kolltveit KM, Tronstad L, Olsen I (2000) Systemic diseases caused by oral infection. Clin Microbiol Rev 13: 547-558. [Ref.]
- Glurich I, Grossi S, Albini B, Ho A, Shah R, et al. (2002) Systemic inflammation in cardiovascular and periodontal disease: comparative study. Clin Diagn Lab Immunol 9: 425-432. [Ref.]
- Slagter KW, Raghoebar GM, Vissink A (2008) Osteoporosis and edentulous jaws. Int J Prosthodont 21: 19-26. [Ref.]
- Xiong H, Peng B, Wei L, Zhang X, Wang L (2007) Effect of an estrogen-deficient state and alendronate therapy on bone loss resulting from experimental periapical lesions in rats. J Endod 33: 1304-1308. [Ref.]
- Payne JB, Zachs NR, Reinhardt RA, Nummikoski PV, Patil K (1997) The association between estrogen status and alveolar bone density changes in postmenopausal women with a history of periodontitis. J Periodontol 68: 24-31. [Ref.]
- Mori G, Brunetti G, Colucci S, Ciccolella F, Coricciati M, et al. (2007) Alteration of activity and survival of osteoblasts obtained from human periodontitis patients: role of TRAIL. J Biol Regul Homeost Agents 21: 105-114. [Ref.]
- Colucci S, Mori G, Brunetti G, Coricciati M, Pignataro P (2005) Interleukin-7 production by B lymphocytes affects the T cell-dependent osteoclast formation in an in vitro model derived from human periodontitis patients. Int J Immunopathol Pharmacol 18: 13-19. [Ref.]
- Pepelassi E, Nicopoulou-Karayianni K, Archontopoulou AD, Mitsea A, Kavadella A, et al. (2012) The relationship between osteoporosis and periodontitis in women aged 45-70 years. Oral Dis18: 353-359. [Ref.]
- Passos JS, Vianna MI, Gomes-Filho IS, Cruz SS, Barreto ML, et al. (2012) Osteoporosis/osteopenia as an independent factor associated with periodontitis in postmenopausal women: a case-control study. Osteoporos Int 37: 1275-1283. [Ref.]
- Gibert P, Tramini P, Sieso V, Piva MT (2003) Alkaline phosphatase isozyme activity in serum from patients with chronic periodontitis. J Periodontal Res 38: 362-365. [Ref.]
- Smit MD, Westra J, Vissink A, Doornbos-van der Meer B, Brouwer, E et al. (2012) Periodontitis in established rheumatoid arthritis patients: a cross-sectional clinical, microbiological and serological study. Arthritis Res Ther 14: R222. [Ref.]
- Erciyas K, Sezer U, Ustün K, Pehlivan Y, Kısacık B, et al. Effects of periodontal therapy on disease activity and systemic inflammation in rheumatoid arthritis patients. Oral Dis 19: 394-400. [Ref.]
- Brito F, Almeida S, Figueredo CM, Bregman R, Suassuna JH, et al. Extent and severity of chronic periodontitis in chronic kidney disease patients. J Periodontal Res 47: 426-430. [Ref.]
- Ardalan MR, Ghabili K, Pourabbas R, Shoja MM (2011) A causative link between periodontal disease and glomerulonephritis: a preliminary study. Ther Clin Risk Manag 7: 93-98. [Ref.]
- Guerra EN, Vianna L, Sobreira MN, de Araújo FN, de Melo NS (2011) Oral manifestations of hyperoxaluria. J Craniofac Surg 22: 2191-2192. [Ref.]
- Thorman R, Neovius M, Hylander B (2009) Clinical findings in oral health during progression of chronic kidney disease to end-stage renal disease in a Swedish population. Scand J Urol Nephrol 43: 154-159. [Ref.]
- Tramini P, Montal S, Valcarcel J (2007) Tooth loss and associated factors in long-term institutionalised elderly patients. Gerodontology 24: 196-203. [Ref.]
- Yoshihara A, Nakamura K, Miyazaki H (2012) The association between renal function and tooth loss in Japanese community-dwelling postmenopausal women. Gerodontology 29: e363-367. [Ref.]
- Al-Nowaiser A, Roberts GJ, Trompeter RS, Wilson M, Lucas VS (2003) Oral health in children with chronic renal failure. Pediatr Nephrol 18: 39-45. [Ref.]
- Brotto RS, Vendramini RC, Brunetti IL, Marcantonio RA, Ramos AP, et al. (2011) Lack of Correlation between Periodontitis and Renal Dysfunction in Systemically Healthy Patients. Eur J Dent 5: 8-18. [Ref.]
- Garcez J, Limeres Posse J, Carmona IT, Feijoo JF, Diz Dios P (2009) Oral health status of patients with a mild decrease in glomerular filtration rate. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 107: 224-228. [Ref.]
- Lee HK, Lee KD, Merchant AT, Lee SK, Song KB, et al. (2010) More missing teeth are associated with poorer general health in the rural Korean elderly. Arch Gerontol Geriatr 50: 30-33. [Ref.]
- Rivas-Tumanyan S, Campos M, Zevallos JC, Joshipura KJ (2012) Periodontal Disease, Hypertension and Blood Pressure Among Older Adults in Puerto Rico. J Periodontol 84: 203-211. [Ref.]
- Rivas-Tumanyan S, Spiegelman D, Curhan GC, Forman JP, Joshipura KJ (2012) Periodontal disease and incidence of hypertension in the health professionals follow-up study. Am J Hypertens 25: 770-776. [Ref.]
- Bonato CF, do-Amaral CC, Belini L, Salzedas LM, Oliveira SH (2012) Hypertension favors the inflammatory process in rats with experimentally induced periodontitis. J Periodontal Res 47: 783-792. [Ref.]
- Franek E, Klamczynska E, Ganowicz E, Blach A, Budlewski T (2009) Association of chronic periodontitis with left ventricular mass and central blood pressure in treated patients with essential hypertension. Am J Hypertens 22: 203-207. [Ref.]
- Angeli F, Verdecchia P, Pellegrino C, Pellegrino RG, Pellegrino G, et al. (2003) Association between periodontal disease and left ventricle mass in essential hypertension. Hypertension 41: 488-492. [Ref.]
- Kanaparthy R, Kanaparthy A, Mahendra M (2012) C-reactive protein as a marker of periodontal disease. Gen Dent 60: e1-5. [Ref.]
- Lu Q, Jin L (2010) Human gingiva is another site of C-reactive protein formation. J Clin Periodontol 37: 789-796. [Ref.]
- Furuta M, Ekuni D, Yamamoto T, Irie K, Koyama R, et al. (2010) Relationship between periodontitis and hepatic abnormalities in young adults. Acta Odontol Scand 68: 27-33. [Ref.]
- Coates EA, Brennan D, Logan RM, Goss AN, Scopacasa B, et al. (2000) Hepatitis C infection and associated oral health problems. Aust Dent J 45: 108-114. [Ref.]
- Yoneda M, Kato S, Mawatari H, Kirikoshi H, Imajo K, et al. (2011) Liver abscess caused by periodontal bacterial infection with Fusobacterium necrophorum. Hepatol Res 41: 194-196. [Ref.]
- Ohyama H, Nakasho K, Yamanegi K, Noiri Y, Kuhara A (2009) An unusual autopsy case of pyogenic liver abscess caused by periodontal bacteria. Jpn J Infect Dis 62: 381-383. [Ref.]
- Tomofuji T, Ekuni D, Sanbe T, Azuma T, Tamaki N, et al. (2009) Effects of improvement in periodontal inflammation by toothbrushing on serum lipopolysaccharide concentration and liver injury in rats. Acta Odontol Scand 67: 200-205. [Ref.]
- Paju S, Scannapieco FA (2007) Oral biofilms, periodontitis, and pulmonary infections. Oral Dis 13: 508-512. [Ref.]
- El Attar MM, Zaghloup MZ, Elmenoufr HS (2010) Role of periodontitis in hospital-acquired pneumonia. East Mediterr Health J16: 563-569. [Ref.]
- Ajonuma LC (2010) A potential role of Chlamydia pneumoniae in the pathogenesis of periodontal disease in adolescents and adults. Int J Adolesc Med Health 22: 213-217. [Ref.]
- Kreutmayer S, Csordas A, Kern J, Maass V, Almanzar G, et al. (2012) Chlamydia pneumoniae infection acts as an endothelial stressor with the potential to initiate the earliest heat shock protein 60-dependent inflammatory stage of atherosclerosis. Cell Stress Chaperones 18: 259-268. [Ref.]
- Komerik N, Akkaya A, Yildiz M, Buyukkaplan US, Kuru L (2005) Oral health in patients on inhaled corticosteroid treatment. Oral Dis 11: 303-308. [Ref.]
- Scannapieco FA, Ho AW (2001) Potential associations between chronic respiratory disease and periodontal disease: analysis of National Health and Nutrition Examination Survey III. J Periodontol 72: 50-56. [Ref.]
- Kucukcoskun M, Baser U, Oztekin G, Kiyan E, Yalcin F (2012) Initial Periodontal Treatment for Prevention of Chronic Obstructive Pulmonary Disease Exacerbations. J Periodontol 84: 863-870. [Ref.]
- Prasanna SJ (2011) Causal relationship between periodontitis and chronic obstructive pulmonary disease. J Indian Soc Periodontol 15: 359-365. [Ref.]
- Leuckfeld I, Obregon-Whittle MV, Lund MB, Geiran O, Bjørtuft Ø, et al. (2008) Severe chronic obstructive pulmonary disease: association with marginal bone loss in periodontitis. Respir Med 102: 488-494. [Ref.]
- Pustorino R, Nicosia R, D'Ambra G, Di Paola M, Brugnoletti O, et al. (1996) The mouth-stomach crossing of Helicobacter pylori. Riv Eur Sci Med Farmacol 18: 183-186. [Ref.]
- Al Asqah M, Al Hamoudi N, Anil S, Al Jebreen A, Al-Hamoudi WK (2009) Is the presence of Helicobacter pylori in dental plaque of patients with chronic periodontitis a risk factor for gastric infection? Can J Gastroenterol 23: 177-179. [Ref.]
- Lee H, Kho HS, Chung JW, Chung SC, Kim YK (2006) Volatile sulfur compounds produced by Helicobacter pylori. J Clin Gastroenterol 40: 421-426. [Ref.]
- Ii H, Imai T, Yaegaki K, Irie K, Ekuni D (2010) Oral malodorous compound induces osteoclast differentiation without receptor activator of nuclear factor κB ligand. J Periodontol 81: 1691-1697. [Ref.]
- Umeda M, Kobayashi H, Takeuchi Y, Hayashi J, Morotome-Hayashi Y, et al. (2003) High prevalence of Helicobacter pylori detected by PCR in the oral cavities of periodontitis patients. J Periodontol 74: 129-134. [Ref.]
- Gunji T, Matsuhashi N, Sato H, Fujibayashi K, Okumura M, et al. (2008) Helicobacter pylori infection is significantly associated with metabolic syndrome in the Japanese population. Am J Gastroenterol 103: 3005-3010. [Ref.]
- Habashneh RA, Khader YS, Alhumouz MK, Jadallah K, Ajlouni Y (2012) The association between inflammatory bowel disease and periodontitis among Jordanians: a case-control study. J Periodontal Res 47: 293-298. [Ref.]
- Brito F, de Barros FC, Zaltman C, Carvalho AT, Carneiro AJ, et al. (2008) Prevalence of periodontitis and DMFT index in patients with Crohn's disease and ulcerative colitis. J Clin Periodontol 35: 555-560. [Ref.]
Download Provisional pdf here
Article Information
Article Type: Research Article
Citation: Haq MW, Tanwir F, Tabassum S, Nawaz M, Siddiqui MF (2015) Association of Periodontitis and Systemic Diseases. Int J Dent Oral Health, Volume1.1: http://dx.doi.org/10.16966/2378-7090.101
Copyright: © 2015 Haq MW, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Publication history:
SCI FORSCHEN JOURNALS
All Sci Forschen Journals are Open Access
