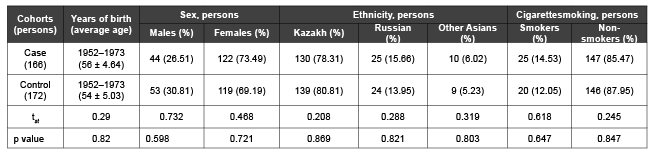
Table 1: Characteristics of control subjects and patients with IHD for analysis of the association between GCLC, GPX4 genes, polymorphisms and IHD
Liliya Skvortsova1* Diana Baizhigitova1 Elmira Khussainova1 Leyla Djansugurova1 Bakhytzhan Bekmanov1 Alma Mansharipova2
1Institute of General Genetics and Cytology, Almaty, Kazakhstan*Corresponding author: Liliya Skvortsova, Kazakhstan Institute of General Genetics and Cytology, al-Farabi st. 93, 050060, Almaty, Kazakhstan, Tel: 8(727)2694616; E-mail: lilia_555@rambler.ru
Background: This study investigated whether the main antioxidant genes GCLM and GPX4 single nucleotide polymorphisms are associated with Ischemic Heart Disease in people living in Kazakhstan.
Methods and Results: We evaluated 166 patients with Ischemic Heart Disease and 172 healthy subjects aged 56 (range 42 – 63). All subjects were genotyped for the presence of GCLM gene promoter polymorphism -588C/Tand GPX-4 718C/T (rs713041) in 3’UTR region.Multiple logistic regression analysis was used to assess differences between age, sex, smoking status and polymorphisms genotyping. In our study GCLM -588TT genotype was significantly associated with the risk of Ischemic Heart Disease (OR=11.75, p=0.02) for general ethnically mixed group; GPX4 718CC geno type also showed a high risk in general group with Ischemic Heart Disease development (OR=2.16, p=0.02).
Conclusions: the polymorphism -588TT of GCLM seems to be a strong risk factor for patients with Ischemic Heart Disease in Kazakhstan.
Oxidative stress; Antioxidants; Ischemic heart disease; Risk factor
Ischemic Heart Disease (IHD) is the most common cause of death in the world, and a major cause of hospital admissions. It is known that IHD is a multifactorial disease with many pathological states. Unlike genetic disorders caused by a defect in a single gene, such diseases are characterized by the mechanism of formation phenotype which is based on the interaction of genetic and environmental factors [1]. Based on the current understanding of the mechanisms of IHD development, there is a group of candidate genes, protein products of which are, or could potentially be involved in the pathogenesis of IHD. Growing evidence shows that high production of free radicals (oxidative stress) and activity of antioxidant proteins play an important role in conditions allowing IHD development. Oxidative stress occurs when there is an imbalance between the production of free radicals and the activity of antioxidant systems in the body [2]. In IHD pathological conditions this leads to the formation of lipid peroxides, endothelial cell components dysfunction, atherosclerotic plaques development and increased platelet aggregation.
In mammalian cells, glutathione and glutathione peroxidases constitute the principal antioxidant defense system [3]. Glutathione is one of the most powerful antioxidant, but is not an essential nutrient, because it can biosynthesized in the body from the amino acids L-cysteine, L-glutamic acid, and glycine. Catalyzing of two-steps ATP-dependent reactions is carried out by two main enzymes: Glutamate Cysteine Ligase (GCL) and Glutathione synthetase (GSS). Bioavailability of the first enzyme is critical for organisms developing and functioning as it’s a rate-limiting enzyme in GSH biosynthesis. GCL is a hetero dimericholoenzyme made up of two different subunits, named a catalytic subunit (GCLC) and a modifier subunit (GCLM).
First subunit GCLC has the main catalytic activity in detoxification reactions, but the Vmax of the reaction is low, and is increased via the addition of the second subunit GCLM. Bough subunits are transcribed from two independent genes, located on distinct chromosomes. Regulation of expression GCL is carried out at the level of transcription of GCLC and GCLM genes, and bought genes contain putative oxidative stressresponsive elements in their promoter/enhancer regions (as antioxidant response elements (AREs)). Several case-control studies indicate possible associations of GCLC and GCLM promoter polymorphisms with cardiovascular diseases development. However, results of these polymorphism-associatedstudies with the development of cardiovascular diseases are uncompleted and contradictory, which can largely be attributed to the ethnic heterogeneity in the distribution and frequency of this polymorphism.
GPx4 isoform is the only one enzyme in the glutathione peroxidases family capable of complex hydroperoxides reduction in phospholipids and low-density lipoproteins and regulates inflammation by affecting cytokine mediated signaling pathways.Polymorphism in the GPx4 gene in a stretch corresponding to the untranslated region on the 3 ‘end of the mRNA is functional and, at the present time, is found in Asian and European populations [4]. There is evidence that endothelial cells from homozygous carriers with polymorphic T allele are more sensitive to the effects of oxidative stress and exhibit elevated levels of monocyte adhesion, which makes them more prone to the development of vascular pathologies.However, many aspects of the relationship of this kind of polymorphism with the development of cardiovascular pathologies, as well as its prevalence in the population are still remain poorly understood and require further studies [5].
Thus, the aim of the present study was to evaluate the association of GCLM and GPX4 polymorphisms with IHD development in Kazakhstan population.
Study subject. To study the role of genetic risk factors with Ischemic Heart Disease developmentpatients from “City Clinical Hospital №1”, cardiology department (Almaty, Kazakhstan) and “Kazakh-Russian Medical University” were selected according to theclinical diagnosis and electrophysiological data of IHD inspected by experienced medical specialists.Blood samples were collected from 166 patients (mean age 56 (range 42–63 years)) with detailed questionnaires. Control blood samples were obtained from 172 healthy individuals matched by age, gender and ethnicity according to the patients group (Table 1). The control group consisted of healthy individuals without clinical manifestations of coronary heart disease, without family history of atherosclerosis and ischemic events at ECG.
DNA was isolated from peripheral blood in the frozen samples (-20°C) by using Genomic DNA Purification Kit» (Fermentas). Detection of qualitative and quantitative characteristics of the DNA samples were measured by spectrophotometry (EppendorfBiophotometer plus).
We studied single nucleotide polymorphisms-588C/T and Leu220Leu (c. 718C/T) forthe GCLM and GPX4 genes respectively. Genotypes of each polymorphism were determined by PCR-RFLP in “Mastersycler” (Eppendorf). 50 ng of target genomic DNA was amplified in 20 µl PCR mixture, containing 10 µl 2 × PCR Master Mix (0.05 U/µL Taq DNA polymerase, reaction buffer, 4 mM MgCl2 , 0.4 mM of each dNTP (Thermo Scientific)) and 5 pM of each specific primer. To amplify the polymorphic Leu220 Leu region of the GPX4, a set of primers we designed GPX4 – 220F 5’GAGAAGGACCTGCCCCACTA3′ and GPX4 – 220R 5′ GTCATGAGTGCCGGTGGAAG3′. For the polymorphic -588°C/T promoter region of the GCLM we used primers and genotyping protocol as described by Nakamura et al. [6]. PCR was carried out with 35 cycles of denaturation 1 min at 95°C, annealing for 30 sec at 61°C (GPX4) or 60°C (GCLM) and extension for 1 min at 72°C. For Leu220Leu (c. 718C/T) detection following 96 bp PCR product was digested by StyI restriction enzyme with subsequent analysis on 8% polyacrylamide gel. TT genotype was identified by the presence of 68-, 28 bp, TC – 96-, 68-, 28 bp and CC – 96 bp bands.
To compare the distribution of variables between case and control cohorts a student’st-test was used. Allele frequencies were calculated according to the standard Hardy-Weinberg equilibrium (HWE). Estimation of the coefficient of relative risk was calculated by the method of “odds ratio” (odd ratio - OR) in conjunction with an estimate 95% confidence interval (95% CI) and the “Chi-square” test (χ2 ) for the degrees of freedom=1.
Statistical analysis of the associations was performed using Software Graph PadInstat™ (V. 2.04. Ralf Stahlman, Purdue University) and “Case-control Study Estimating Calculator” from gene Expert Company (“GosNIIGenetika” State Scientific Centre of Russian Federation, http:// gen-exp.ru/calculator_or.php).
There were no significant differences in age, gender, ethnicity and smoking status between patients and control groups.
Genotype frequencies for GCLM -588C/TandGPX4 Leu220Leu (c. 718C/T) are shown in Figure 1. The genotype distributions of the studied polymorphisms were in HWE as among the controls as among the IHD patients. To address the hypothesis that known functional variants in antioxidant genes affect IHD risk the association of the individual SNPs with IHD risk was assessed by evaluating the data using general, recessive and dominant models. Taking into account the ethnic heterogeneity of groups statistical analysis of the association of each type of polymorphism with the IHDdevelopment was carried out as for the whole groups as for groups according to the mainethnic groups.
Table 2 shows the relative risk impact of polymorphisms GCLM and GPX4 on the development of IHD in general population and main ethnic groups in Almaty (Kazakhstan) calculated on the general inheritance model.
According to the multiple general model in all ethnic groups GCLM -588TT genotype is significantly associated with risk of IHD (OR=11.75). This is confirmed by dominant model where genotype TT versus CC+CT also has a high association risk (OR=11.75, 95%CI=0.64 – 214.19, χ2 =5.26, p=0.02).
According to the separate analysis of the main ethnic groups it was revealed that GCLM -588TT genotype strongly associated with increased risk of IHD in Russians (OR=10.32, CI 95%= 0.52–203.37, χ2 =4.2, p=0.12) especially in recessive model (OR=10.32, χ2 = 4.19, p=0.04); whereas for Kazakhs this genotype was not present in studied group and didn’t show any associations in different models. Furthermore, higher risk of IHD is determined statistically significant for -588CC genotype in Kazakhs only according to the recessive model (OR=1.59, χ2 =3.45, p=0.06).
Our statistical data demonstrates high association of GPX4 718CC genotype in general group with IHD development (OR=2.16, CI 95%=1.25–3.73, χ2 =8.05, p=0.02), what is considered by the recessive inheritance model with strong significance level (OR=2.16, χ2 =7.78, p=0.005). The general model of inheritance also shows that GPX4 718TC has a protective effect (OR=0.53) but not for the TT genotype (OR=1.05). Similar results were obtained in analysis of the Kazakh group where GPX 718CC genotype also had a high risk status with IHD according to the general (OR=2.48, CI 95%=1.32–4.66, χ2 =8.22, p=0.02) and recessive models (OR=2.48, χ2 =8.22, p=0.004). Opposite, in Russians the TT genotype was turned out to be the higher IHD risky genotype (OR=2.88, CI 95%=0.11–74.24, χ2 =0.95, p=0.62 dominant model; OR=2.88, χ2 =0.94, p=0.33 recessive model) but these data is not statistically significant.

Table 1: Characteristics of control subjects and patients with IHD for analysis of the association between GCLC, GPX4 genes, polymorphisms and IHD

Figure 1: Frequency of polymorphisms’ genotypes: a) for Leu220Leu (c. 718C/T) of the GPX4 gene (control χ2 =57.62, p=0; case χ2 =22.35, p<0.0001), and b) for-588C/T of the GCLM gene (control χ2 =16.31, p=0.00005; case χ2 =1.43, p=0.23) in all ethnics groups between IHD patients and healthy group.
In 2002 Nakamura et al. [6] identified two promoter polymorphisms (5′-flanking region) of GCLM the -588 C/T and 23 G/T which are completely linked. They also showed the -588C/T polymorphism has a functional effect on expression of this gene to oxidative stress, in particular -588T allele carriers had a lower level of serum GSHwhichcould be possibly explained by the observation of suppression of the GCLM mRNA expression in -588CT cells compare to -588CC. Their case-control study revealedthat -588T allele may be an independent risk factor for MI in Japanese population [6]. Their further studiesdemonstrated a negative effect of -588T polymorphism on endothelium-dependent vasomotor reactivity in large and resistance coronary arteries (abnormally low dilation effect or high constriction) and may affect to coronary artery disease development thought impaired GSH synthesis in vascular cells to oxidative stress and thus to increase of susceptibility to impairment of NO-mediated endothelial vasomotor function [7]. There is a small amount of further reports in the literature about role of this polymorphism in cardiovascular diseases development. Katakami et al. [8] showed -588T genotype is significantly associated with carotid intima-media thickness in type 2 diabetic patients, as a strong predictor to atherosclerosis and further IHD and MI development [8]. But, no effect of this polymorphism on MI in type 2 diabetes was found in Nao to Katakami et al. [9]. Also Muehlhause et al. [10] didn’t find association of C-588T polymorphism with the risk and extent of IHD in a German cohort [10]. It emphasizes that the associative analysis is highly dependent on ethnicity, age, allele frequency, type of disease and role of antioxidant enzymes in each disease.
In our ethnically mixed case-control study we showed -588TT variant has a higher risk for IHD (OR=11.75). Detailed analysis of each ethnic group separately revealed that this tendency takes place onlyfor Russians (the TT OR=10.32, especially for TT versus CC+CT), but these data is not statistically significant that may be explained by the limited and small number of this group in mixed population (case (n)=25, control (n)=24). For the Kazakhs this association wasn’t observed, that accentuates ethnically genetic specificity in one study.
According to the different earlier studies of GPX4 gene, a synonymous polymorphism Leu220Leu (c. 718C>T) demonstrates a functional consequences; it is proposed that it could affect distinct protein binding
properties to the 3′ untranslated region (3′UTR) of the GPX4 mRNA (within the selenocysteine insertion sequence (SECIS)) and thus modulate Gpx4 synthesis though forming GPX4mRNA-proteins complexes [11]. First studies proven it functionally on leukotriene (LT) biosynthesis though measurement of 5-lypooxygenase (5-LOX) metabolites in leucocytes with different 718C/T genotypes. 718C variant had increased levels of LT compare to 718T and 718T/C [12]. Later similar results were obtained for epidermoid carcinoma tumor cell line [13]. This confirms GPX4 mRNA
(3′UTR) stem-loop region to up regulate arachidonate metabolism, important for LT synthesis and subsequent inflammation reaction development, including vessels vasodilatationwhichplays a significant role in vessels epithelium damaging and atherosclerosis development. As, GPX4 is a selenium dependent, there was noticed that the level of selenoprotein and its mRNA in 718C/T carriers are differentially affected by Se supply and type of the cells; in lymphocytes homozygous for the C variant showed more stable concentrations of the Gpx4 in cells than T-variant after cessation of the Se diet; whereas T carriers have less sensitive response to Se diet, whereas for endothelial cells the data showed opposite results [14,15]. A few case-control reports showed possible associations of this polymorphism with the diseases development: 718C has an increased risk of death in breast cancer [16,17], colorectal cancer [18] and cerebral stroke (CS) in patients having essential hypertension (EH) [19]. But these data seems to be conflictive to GPX4 functional data research: been active and increasing LOX-5 metabolism 718C variant demonstrates higher association risk to the diseases. In our case-control study genotype 718CC also demonstrated a higher risk for HID in general population (OR=2.16, CI 95%=1.25–3.73, χ2 =8.05, p=0.02), especially in recessive inheritance model (OR=2.16, CI 95%=1.25–3.73, χ2 =7.78, p=0.005). Separate ethnic analysis showed different associations for Kazakhs and Russians. For Kazakhs 718CC genotypeturned out to be an associative significant for IHD in general (OR=2.48, CI 95%=1.32–4.66, χ2 =8.22, p=0.02) and recessive models (OR=2.48, CI 95%=1.32–4.66, χ2 =8.22, p=0.004). For Russians analysis didn’t reveal any statistically significant associations with IHD (for TT genotype OR=2.88, CI 95%=0.11–74.24, χ2 =0.95, p=0.62), perhaps because of small number of this group and further studies needed on large case-control group.
In conclusion, the present data demonstrate that two SNPs in the antioxidant genes GCLM -588TT and GPX-4 718CC are associated with IHD in Kazakhstan mixed population and may prove earlier studies on important functional role of these genes’ SNP in cardiovascular disease development. But these data also shows ethnically heterogeneity association and need to be more detailed study on larger groups of each ethnic cohort separately. However, the present case-control study has limitations. Firstly, pathogenesis of IHD is very complex with involvement of different antioxidant systems in organism and the specific action mechanism of the GCLM and GPX4 enzymes in this disorder is far from clear. Secondly, no data on the additional lipid biochemical parameters were available for the analysis (low density lipoprotein cholesterol (LDL-C), high density lipoprotein cholesterol (HDL-C), and triglycerides (TG)); patients were selectedaccording to the clinical manifestations of IHD and electrophysiological data, without coronary angiography of atherosclerosis stage development. Moreover, in this study we did not consider in patients such comorbidity diseases like hypertension, diabetes and others. Thirdly, current sample size allowed only a limited power to detect interactions and more expanding sample size need to further investigate genetic influence of the studied polymorphism on the development of IHD. Besides, other GCLM and GPX4 genes’ polymorphisms may affect the GCLM and GPX4 enzymes activity and their additional influence on pathogenesis of IHD.

Table 2: Association between the GCLM and GPX4 polymorphisms and IHD in a case-control study
Download Provisional PDF Here
Article Type: Research Article
Citation: Skvortsova L, Baizhigitova D, Khussainova E, Djansugurova L, Bekmanov B, et al. (2015) Polymorphisms in Antioxidant Genes GCLM and GPX-4 and Ischemic Heart Disease Development in Kazakhstan Population. Clin Res Open Access 1(1): doi: http://dx.doi.org/10.16966/2469-6714.104
Copyright: © 2015 Skvortsova L, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Publication history:
All Sci Forschen Journals are Open Access