
Figure 1: Clinical Photographs of a euchromatic, translucent papule lesion in the lateral canthus of right eye.


MC Cuéllar-Martinez1 Vanessa García-Valencia2* C Montoya-Bueno2 R Restrepo-Molina3
1Dermatologist, Specialist in Dermatopathology Universidad CES, Medellin, Colombia*Corresponding author: Vanessa García Valencia, Pathologist, dermatopathology, CES University, Medellin, Colombia, Phone: (+57) 311-645-6550; E-mail: vanessagava@hotmail.com
Endocrine Mucin-Producing Sweat Gland Carcinoma (EMPSGC) is a rare, low-grade, malignant tumor derived from sweat glands, arising preferentially in skin from the periorbital region. EMPSGC tumors are more frequently found in elderly female patients. To date, only around 70 cases have been reported in the literature [1-3]. Therefore, thorough immunohistochemical characterization of EMPSGC tumors is of pivotal importance to confirm its diagnosis [4-6].
Sweat gland neoplasms/pathology; Eyelid neoplasms/pathology; Endocrine mucin-producing sweat gland carcinoma; Neuroendocrine differentiation
A 63-year-old female patient presented to our clinic with a severalmonth history of an asymptomatic lesion in the lateral canthus of her right eye. Upon physical examination, a euchromatic, translucent papule was observed (Figure 1). An incisional biopsy was performed, and samples were submitted for histopathological analysis, under a suspected diagnosis of eccrine hidrocystoma [7-9].

Figure 1: Clinical Photographs of a euchromatic, translucent papule lesion in the lateral canthus of right eye.
Histological sections showed a pseudoencapsulated lesion, composed by a cystic area covered by cuboidal epithelium (Figure 2A), with a solid area containing monomorphic cells, foci of glandular differentiation (Figure 2B), and other cells of apocrine appearance exhibiting decapitation secretion (Figure 2C). Immunohistochemical analysis showed positive staining for Cytokeratin 7 (CK7) (Figure 3A), chromogranin (Figure 3B), Neuron-Specific Enolase (NSE) (Figure 3C), and Estrogen Receptor (ER) (Figure 3D), synaptophysin (Figure 3E). On the other hand, the lesion was negative for CEA, P63 (Figure 3F), and CD138 and presented weak Ki67 staining (Figure 3G).
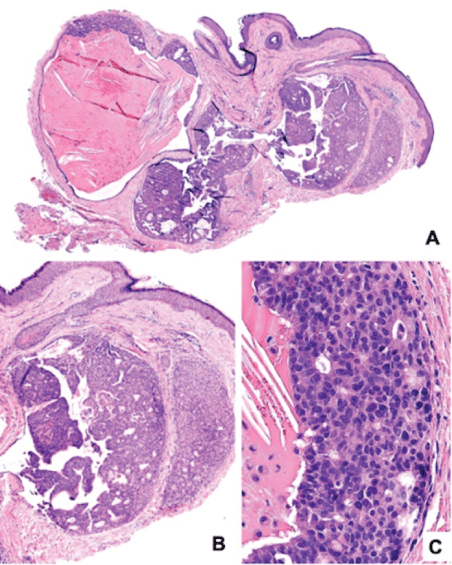
Figure 2: Histological micrographs of EMPSGC hematoxylin-eosin stained A. Dermic tumor (H&E, 4x) B. Dermic tumor showing cystic, solid, circumscribed region (10x), C. Monomorphic cells with glandular differentiation, and formation of pseudorosettes (40x).

Figure 3: Immunohistochemistry A. Dermic tumor cells showing positive CK7 expression (4x), B. Dermic tumor cells showing positive chromogranin expression (4x), C. Dermic tumor cells showing positive SNE expression by immunohistochemistry (4x), D. Representative images of dermic tumor cells showing positive estrogen receptor expression by immunohistochemistry (4x), E. Representative images of dermic tumor cells showing positive synaptophysin expression by immunohistochemistry (4x) F. Representative images of dermic tumor cells showing lack of p63 expression by immunohistochemistry (10x) G. Representative images of dermic tumor cells showing moderately positive Ki67 expression by immunohistochemistry (10x).
Taking into account these findings, an endocrine mucin-producing sweat gland carcinoma was diagnosed. The patient underwent complete surgical resection of the lesion [10-12].
Endocrine Mucin-Producing Sweat Gland Carcinoma (EMPSGC) is a rare neoplasm, first described in 1997 [13,14], low-grade primary tumor, that is slow growing, and of low recurrence. Its morphology and immunophenotype is similar to that of lesions of the breast including endocrine Ductal Carcinoma In Situ (DCIS) and solid papillary carcinoma [3,13]. While (EMPSGC) usually affects older adults with a predominance in women and has a predilection for the skin of the eyelid [13]. Rare cases involving such as cheeks, periauricular region, occipital area, scalp, and even thorax have also been reported [3,4,15,16]. Although its clinical course is generally asymptomatic, it appears to be a precursor to invasive mucinous carcinoma with the potential for local recurrence [13-15]. Zembowicz A, et al. describe the lower eyelid to be more frequently involved as compared to the upper; Hoguet A, et al. report otherwise [14,17].
Clinically, this lesion presents as slow-growing, nodular, or pedunculated, of variable color, associated to the presence of telangiectasias or madarosis, being more frequent in patients with an average age of 67.5 years [3,13,14]. Dermoscopic examination shows pink ovoid nests of cobblestone appearance, among which are blue or red globules separated by a whitish-pink mesh [5]. EMPSGC is a wellcircumscribed, multinodular tumor with solid and cystic regions, of papillary and cribriform architectural subtypes, and localized in the dermis, although cases with focal connections to the epidermis have also been described [3]. EMPSGC cells are oval or polygonal, with a pale eosinophilic cytoplasm, with peripheral palisades, and presence of perivascular pseudorosettes [6-8].
EMPSGC presents nuclear polymorphism that ranges from mild to moderate; salt-and-pepper chromatin, as well as mitotic activity and occasionally foam cells are also present [6-9]. Usually, significant mucin deposits are not observed, however, if present, a mucinous carcinoma should be suspected [6].
Immunohistochemical profile of EMPSGC includes positive staining for neuroendocrine markers such as synaptophysin, SNE, chromogranin A, CD57, and CD56 [6,7,10]. In addition, tumor cells are positive for CAM5.2, gross cystic disease fluid protein 15 (GCDFP-15), WT1, Ber-Ep4, estrogen receptor, and progesterone receptor [3,6-8]. Positive staining for CK7 may lead to confusion at time of differential diagnosis, since metastatic breast cancer is histologically similar to EMPSGC. Calponin, p63, CD10, and Smooth Muscle Actin (SMA) must be carefully interpreted since these are positive markers for periadnexal mesenchymal cells (CD10) and Stromal Myofibroblasts (SMA) surrounding the tumor, giving the impression of an intact layer of myoepithelial cells.
Altogether, our findings and those described by Dhaliwal CA, et al., indicate that this lesion should be considered as an invasive tumor [6]. Additional biomarkers have been recently explored in EMPSGC; positive GATA3 staining has been described in a case report [11] and strong nuclear MYB staining, especially in mucin-poor EMPSGC tumors, has been reported in a case series [3]. In the latter, the authors propose that this tumor might not represent a precursor lesion of Primary Cutaneous Mucinous Carcinoma (PCMC) due to their previously described negative MYB expression. However, cases in which EMPSGC and PCMC coexist have also been reported [3].
Histologic differential diagnosis of endocrine mucin-producing salivary gland carcinoma include hidradenoma, hidradenocarcinoma, basal cell carcinoma, apocrine adenoma, metastatic endocrine ductal breast carcinoma, and primary cutaneous mucinous carcinoma [1-3].
Unlike papillary carcinoma of the breast, and mucinous tumors of lung or pancreas, EMPSGC tumors have not been associated with mutations in PIK3CA, AKT1, KRAS, GNAS, or EGFR genes. Therefore, accurate EMPSGC diagnosis may also be achieved by performing molecular studies [12]. Cutaneous metastatic lesions of endocrine ductal breast carcinoma are usually identified when the disease is locally advanced; thus, the correlation between clinical and paraclinical parameters plays a key role in discriminating these lesions from EMPSGC, since both types of lesions exhibit positive staining for estrogen receptor, progesterone receptor, and CK7 [6].
EMPSGC is a neoplasm of asymptomatic nature; although recurrences have been described, no metastases have been reported [13,14].
Finally, the goal of EMPSGC treatment is to reduce tumor recurrence, improve esthetic results, and functional sequelae [1]. Emmanuel P, et al. [18-20] described a case of GCPSMS in a 65-yearold woman in whom the tumor recurred 3 years after excision. For this reason, complete surgical excision and clinical follow-up for a period of at least 24 months have been suggested and clinical followup are recommended, as the tumor is at risk of local recurrence and progression to invasive mucinous carcinoma [19]. Although Mohs micrographic surgery could be considered, a traditional surgical resection with an adequate margin also produces successful results [13,14,18].
We report a new case of EMPSGC for which we describe tumor morphology and immunohistochemical profile. Differential diagnosis of this tumor includes benign neoplasms and cutaneous metastases. Therefore, because EMPSGC is clinically and histologically similar to benign neoplasms, accurate diagnosis can be achieved by performing the studies herein described in a timely manner.
Contributed to sample acquisition, evaluated all aspects of the current work in order to verify data accuracy and ethical compliance, participated in manuscript writing, and approved final version of this manuscript. Conceived and designed this manuscript, contributed to sample acquisition, evaluated all aspects of the current work in order to verify data accuracy and ethical compliance, participated in manuscript writing, critically reviewed intellectual content, and approved final version of this manuscript.
The authors have no conflict of interest to declare.
Download Provisional PDF Here
Aritcle Type: CASE REPORT
Citation: Cuéllar-Martinez MC, García-Valencia V, Montoya-Bueno C, Restrepo-Molina R (2022) Endocrine Mucin-Producing Sweat Gland Carcinoma: A Case Report and Literature Review. J Clin Cosmet Dermatol 6(2): dx.doi.org/10.16966/2576-2826.171
Copyright: © 2022 Cuéllar-Martinez MC, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Publication history:
All Sci Forschen Journals are Open Access