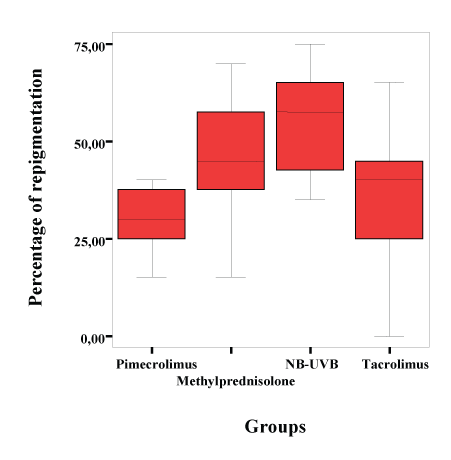
Figure 1: Percentage of Repigmentation at 24 week.


Selma Bakar Dertlioğlu*
Department of Dermatology, Dermatology Clinic, Elazığ, Turkey*Corresponding author: Selma Bakar Dertlioğlu, Department of Dermatology, Dermatology Clinic, Elazığ, Turkey, E-mail: selmadertlioglu@hotmail.com
Background: The treatment of vitiligo is currently very difficult.
Aims: The aim of the present study was to determine the efficacy and safety of narrow-band UVB (NB-UVB), topical pimecrolimus, topical tacrolimus and topical methylprednisolone aceponate in the treatment of vitiligo.
Methods: A total of 88 patients with vitiligo were enrolled in this study. The patients were divided into four groups: NB-UVB phototherapy (22), 1% pimecrolimus cream (22), 0.1% tacrolimus ointment (22), methylprednisolone aceponate cream (22).
The activity of each vitiligo lesion was assigned a VIDA score from -1 to +4 at baseline, as well as 2,4,8,12, and 24 weeks for the whole period of therapy. The patients were examined using digital photographs, and the measurement of the efficacy of treatment was based on the percentage and score of repigmentation of all scored lesions.
Results: In the 4th treatment week, the methylprednisolone aceponate group was found to have significantly higher repigmentation percentages and scores compared with the pimecrolimus group (p=0.023, p=0.021, respectively). In the 8th week of treatment, the highest repigmentation percentage was obtained in the NB-UVB group. The most robust treatment responses in the patients with acral involvement were achieved with the NB-UVB and methylprednisolone aceponate therapies; in the patients with generalized involvement, the most robust treatment responses were achieved with the NB-UVB therapy.
Conclusion: Methylprednisolone aceponate produced a prompt treatment response, but both methylprednisolone aceponate and NB-UVB treatment had the same long-term efficacy and produced more robust responses than pimecrolimus and tacrolimus.
Methylprednisolone aceponate; Narrow-band UVB; Pimecrolimus; Tacrolimus; Vitiligo
Both methylprednisolone aceponate and NB-UVB treatment had the same efficacy over the long-term and produced better responses than pimecrolimus and tacrolimus.
Vitiligo is an acquired, idiopathic disorder that is characterized by depigmented macules that result from damage to and destruction of melanocytes [1]. Vitiligo affects between 0.5 and 2% of the general population causing cosmetic and psychosocial problems [2]. The treatment of vitiligo is often stressful and unsatisfying and remains a challenge for dermatologists, although a wide range of therapeutic options have been proposed and are currently available. The mainstays of vitiligo therapy include the application of potent topical corticosteroids and the administration of phototherapy, including either psoralen-UVA (PUVA) or NBUVB [3-5]. Topical calcineurin inhibitors are another option that has been recently introduced for the treatment of vitiligo; these compounds offer the advantage of prolonged use while avoiding the adverse events related to the long-term use of topical steroids. Topical immunomodulators include 0,1% and 0,03% (Protopic) tacrolimus ointment and 1% pimecrolimus cream (Elidel) [6,7]. The objective of the present study is to compare and contrast NB-UVB, pimecrolimus, tacrolimus and methylprednisolone aceponate treatments and to investigate the efficacy, side effects and practicality of traditional methods and more contemporary treatments as well as the efficacy and side effects of treatment methods based on the area involved.
The study included 88 patients who were diagnosed with vitiligo through clinical and Wood’s light examinations. The patients were referred to our dermatology outpatient clinic between April 2009 and April 2010.
The exclusion criteria were as follows: pregnancy or lactation, infections, neurological or psychiatric disorders, autoimmune disease (systemic lupus erythematosus, dermatomyositis, multiple sclerosis, or Graves’ disease), immune defects, heart disease, kidney failure, previous or current history of neoplasms. For patients who were administered any local or systemic immunosuppressive therapy, a washout period of at least 6 months was required. Patients who were scheduled to receive phototherapy and had a history of previous side effects or phototoxic reactions related to phototherapy, a history of photosensitivity or photomediated disorders and claustrophobia were excluded. Informed written consent was obtained from patients after the nature of the treatment was carefully explained, including details of its possible benefits and side effects. The wash out phase for current treatment was 24 weeks.
At baseline and at weeks 2, 4, 8, 12, and 24, the patients were examined with digital photography. Then, two dermatologists independently evaluated the photographs and compared them with the baseline photographs. The measurement of the efficacy of treatment was based on the percentage and score of repigmentation averaged for all of the lesions.
The patients were clearly informed about their disease, possible treatment options, possible side effects and the study plan. Each patient provided signed informed consent for the treatment and the photos. No conflicts of interest concerning sponsorship of any type was noted in this study.
The patients were scheduled on the basis of a computer-generated randomization into four groups: 22 patients received NB-UVB phototherapy 3 times a week, 22 patients were treated with 1% pimecrolimus cream b.i.d., 22 patients applied 0.1% tacrolimus ointment b.i.d. and 22 patients were treated with methylprednisolone aceponate cream b.i.d. All four treatment regimens were performed for 24 weeks.
The initial NB-UVB dosage was determined before the treatment according to the minimal erythema dosages per patient. The minimal erythema dosages were tested on one of the patient’s vitiligo patches. NB-UVB dose increments were regulated with regard to the schedule used in our clinic. The starting dose was generally 0,06 J/cm2 to 0,08 J/cm2 depending on the skin type and the percentage of disease involvement; the dose was increased by 0,01 J/cm2 each treatment. All patients were treated with NB-UVB as monotherapy using a Waldman UV 7001 K unit as the light source for NB-UVB, containing a bank of forty fluorescent tubes (Philips TL-01) with an emission spectrum of 310-315 nm, and a maximum wavelength of 311 nm. The treatment duration was 24 weeks, and the frequency was three times a week on nonconsecutive days. During each treatment, the affected parts were exposed, and the genital area was shielded. The optimal constant dose was achieved when minimal erythema occurred in the lesions. If symptomatic erythema (burning, pain) or blistering developed, the irradiation dose was decreased by 20%. During treatment, the eyes were protected with UV-blocking goggles. If significant depigmentation was present on the eyelids and patients insisted on treating these areas, they were told to keep their eyes closed during treatment. Patients were advised to apply sunscreen on the exposed areas and to protect their skin from excessive sun exposure. The study was approved by the local ethics committee. Patients signed informed consent to the procedure in compliance with Helsinki declaration of 1964 (revised 2013) [8].
The 1% pimecrolimus group received 1% pimecrolimus cream b.i.d for 24 weeks. The 0.1% tacrolimus group received 0.1% tacrolimus ointment b.i.d for 24 weeks. The methylprednisolone aceponate group received intermittent therapy over the 24 weeks; the methylprednisolone aceponate cream was administered b.i.d on weekdays, and the treatment was discontinued on weekends.
There was no significant difference among the groups in terms of age, sex, duration of disease, involvement percentage at the onset of the disease, and onset VIDA (Vitiligo Disease Activity) score (p>0.05) (Table 1).
| Methylprednisolone aceponate group (n=19) | Pimecrolimus group (n=20) | Tacrolimus group (n=21) | UVB group (n=20) | |
| Men (n, %) | 10 (52.6) | 6 (30.0) | 7 (33.3) | 5 (25) |
| Women (n, %) | 9 (47.4) | 14 (70.0) | 14 (66.7) | 15 (75) |
| Age range (years) | 7-46 | 9-42 | 10-40 | 10-32 |
| Age* | 25.05 ± 11.4 | 19.25 ± 11.3 | 21.28 ± 9.3 | 18.95 ± 7.5 |
| Disease duration* | 5.89 ± 3.33 | 5.90 ± 4.29 | 6.90 ± 3.25 | 6.35 ± 3.32 |
| Acral involvement | 6 (31.6) | 4 (20.0) | 3 (14.3) | 2 (10.0) |
| Generalized involvement | 12 (63.2) | 15 (75.0) | 18 (85.7) | 18 (90.0) |
| Segmental involvement | 1 (5.3) | 1 (5.0) | 0 | 0 |
Table 1: Demographic and clinical data of patients with vitiligo.
*(Mean ± SD)
The activity of each vitiligo lesion was graded as a VIDA score from -1 to +4 at baseline. This scoring system is based on the patient’s opinion of the disease activity within the time periods indicated as follows: active in the past 6 weeks (score +4); active in the past 3 months (score +3); active in the past 6 months (score +2); active in the past year (score +1); stable for at least 1 year (score 0); and stable for at least 1 year with spontaneous repigmentation (score -1). The term “active” is defined as the expansion of existing lesions or the appearance of new lesions. “Stable” refers to the condition when these symptoms are not present [9].
At baseline and at weeks 2, 4, 8, 12, and 24, the patients were examined with digital photographs. Then, two dermatologists independently evaluated the photographs and compared them with the baseline photographs. The measurement of the efficacy of treatment was based on the percentage and score of repigmentation averaged for all of the lesions. The score of repigmentation (or improvement) was graded as follows [10]:
Side-effects, such as pruritus, burning sensation and erythema were recorded at weeks 2, 4, 8, 12, and 24 of therapy and were graded as mild, moderate or severe using a four-point scale (0, absent; 1, mild; 2, moderate; 3, severe).
SPSS version 12.0 was used for the statistical analyses. Values obtained in the study are presented as the mean ± SD. Kruskal-Wallis and Mann-Whitney U tests were used for the comparison between groups. Values for which p<0.05 were considered to be statistically significant.
The study registered a total of 88 vitiligo patients, 22 in each group. Two patients in the pimecrolimus group, 2 patients in the NB-UVB group, 1 patient in the tacrolimus group and 3 patients in the corticosteroid group were excluded from the study as a result of treatment noncompliance. Twenty patients in the pimecrolimus group, 20 patients in the NB-UVB group, 21 patients in the tacrolimus group and 19 patients in the corticosteroid group completed the study. No statistically significant differences were detected in the demographics, baseline characteristics, medical history and clinical examination between the four groups (Table 1).
According to the classification of sun-reactive skin types, 19 patients showed skin phototype II, and 61 patients showed skin phototype III. The wash out phase for current treatment was 24 weeks.
In the 2 week of UVB treatment, there was no improvement in the group receiving pimecrolimus and NB-UVB treatment; however, limited improvement was observed in the patients who received methylprednisolone aceponate and tacrolimus treatment (Table 2).
| Methylprednisolone aceponate group | Pimecrolimus group | Tacrolimus group | Narrow-band UVB group | ||
| Baseline | VIDA score* | 1.63 ± 0.68 | 1.60 ± 0.68 | 1.61 ± 0.66 | 1.55 ± 0.51 |
| Percentage of involvement* | 51.21 ± 23.30 | 53.60 ± 25.03 | 61.28 ± 19.08 | 64.30 ± 17.43 | |
| Vitiligo activity score* | 1.63 ± 0.68 | 1.60 ± 0.68 | 1.61 ± 0.66 | 1.55 ± 0.51 | |
| 2nd week | Percentage of Repigmentation* | 0.78 ± 2.50 | 0 |
0.23 ± 1.09 | 0 |
| Score of repigmentation* | 0.10 ± 0.31 | 0 |
0.46 ± 0.21 | 0 |
|
| 4th week | Percentage of Repigmentation* | 4.47 ± 5.74a | 1.00 ± 3.07a | 1.90 ± 4.02 | 2.50 ± 4.72 |
| Score of repigmentation* | 0.42 ± 0.50b | 0.10 ± 0.30b | 0.19 ± 0.40 | 0.20 ± 0.41 | |
| 8th week | Percentage of Repigmentation* | 13.42 ± 10.80c | 6.50 ± 10.27c,d | 8.80 ± 7.40 | 14.50 ± 10.99d |
| Score of repigmentation* | 0.84 ± 0.60 | 0.50 ± 0.68e | 1.05 ± 0.60 | 1.14 ± 2.10e | |
| 12th week | Percentage of Repigmentation* | 22.89 ± 12.83 | 17.75 ± 13.12f | 16.95 ± 8.91g | 27.25 ± 11.75f,g |
| Score of Repigmentation* | 1.31 ± 0.67 | 1.00 ± 0.64 | 1.35 ± 0.58 | 1.80 ± 3.07 | |
| 24th week | Percentage of Repigmentation* | 45.52 ± 15.44h,ı,j | 31.25 ± 14.49h | 33.85 ± 16.09j,k | 55.25 ± 12.61ı,k |
| Score of Repigmentation* | 2.21 ± 0.63l,m,n | 1.75 ± 0.71l | 2.65 ± 0.48n,o | 2.85 ± 5.12m,o |
Table 2: Responses of groups to treatment at the onset, and weeks 2nd, 4th, 8th, 12th and 24th.
*(Mean ± SD)
a: p=0.02, methylprednisolone aceponate vs pimecrolimus
b: p<0.02, methylprednisolone aceponate vs pimecrolimus
c: p=0.04, methylprednisolone aceponate vs pimecrolimus
d: p=0.02, narrow-band UVB vs pimecrolimus e: p=0.01, narrow-band UVB vs pimecrolimus
f: p=0.02, narrow-band UVB vs pimecrolimus
g: p=0.003, narrow-band UVB vs tacrolimus
h: p=0.005, methylprednisolone aceponate vs pimecrolimus
ı: p=0.03, narrow-band UVB vs methylprednisolone aceponate
j: p=0.02, methylprednisolone aceponate vs tacrolimus
k: p<0.001 narrow-band UVB vs tacrolimus
l: p=0.04, methylprednisolone aceponate vs pimecrolimus
m: p=0.02, narrow-band UVB vs methylprednisolone aceponate
n: p=0.02 tacrolimus vs methylprednisolone aceponate
o: p<0.001 narrow-band UVB vs tacrolimus
In the 4 Treatment week, the methylprednisolone aceponate group was found to have significantly higher repigmentation percentages and scores compared with the pimecrolimus group (p=0.023 and p=0.021, respectively). At weeks 8, 12, 24 of treatment, the highest repigmentation percentage was obtained in the NB-UVB group. There was no difference in the improvement scores of the pimecrolimus and tacrolimus groups at weeks 2, 4, 8, 12, and 24 of treatment (p>0.05) (Table 2) (Figure 1).

Figure 1: Percentage of Repigmentation at 24 week.
Treatment responses of all the patients in the study were also compared in terms of the type of involvement. The most robust treatment responses in the patients with acral involvement were found in the NB-UVB group at weeks 2,4,8, and 12 (Table 3).
| Pimecrolimus group | Methylprednisolone aceponate group | Narrow-band UVB group | Tacrolimus group | ||
| 2nd week | Percentage of Repigmentation* | 0 | 0 | 0 | 0 |
| Score of Repigmentation* | 0 | 0 | 0 | 0 | |
| 4th week | Percentage of Repigmentation* | 2.50 ± 5.00 | 3.33 ± 5.15 | 5.00 ± 7.07 | 0 |
| Score of Repigmentation* | 0.25 ± 0.50 | 0.33 ± 0.51 | 0.50 ± 0.70 | 0 | |
| 8th week | Percentage of Repigmentation* | 7.50 ± 15.00 | 11.66 ± 8.75 | 20.00 ± 14.14 | 3.33 ± 5.77 |
| Score of Repigmentation* | 0.50 ± 1.00 | 0.83 ± 0.40 | 1.50 ± 0.70 | 0.33 ± 0.57 | |
| 12th week | Percentage of Repigmentation* | 21.25 ± 13.14 | 20.00 ± 11.40 | 30.00 ± 14.14 | 12.00 ± 9.84 |
| Score of Repigmentation | 1.25 ± 0.50 | 1.16 ± 0.40 | 1.50 ± 0.70 | 1.00 ± 0.00 | |
| 24th week | Percentage of Repigmentation* | 37.50 ± 18.48 | 37.50 ± 15.08a | 62.50 ± 3.53a,b | 20.33 ± 19.50b |
| Score of Repigmentation* | 2.00 ± 0.83 | 2.00 ± 0.63c | 3.00 ± 0.00c | 1.33 ± 0.57 |
Table 3: Weekly treatment responses of patients with vitiligo lesions on acral areas.
*(Mean ± SD)
a: p=0.09, narrow-band UVB vs methylprednisolone aceponate
b: p=0.03, narrow-band UVB vs tacrolimus
c: p=0.01, narrow-band UVB vs methylprednisolone aceponate
Among the vitiligo patients with generalized involvement, the most robust treatment response in the fourth week was observed in the methylprednisolone aceponate group, and the efficacy of topical corticosteroids was observed earlier than the other treatments. The most robust responses in the patients with generalized involvement at weeks 12 and 24 were achieved in the methylprednisolone aceponate and NB-UVB groups (Table 4).
| Pimecrolimus group | Methylprednisolone aceponate group | Narrow-band UVB group | Tacrolimus group | ||
| 2nd week | Percentage of Repigmentation* | 0 | 1.25 ± 3.10 | 0 | 0 |
| Score of Repigmentation* | 0 | 1.16 ± 0.38 | 0 | 0 | |
| 4th week | Percentage of Repigmentation* | 0.66 ± 2.58a | 5.41 ± 6.20a | 2.22 ± 4.60 | 2.22 ± 4.27 |
| Score of Repigmentation* | 0.50 ± 0.52b | 0.66 ± 0.25b | 0.16 ± 0.38 | 0.22 ± 0.42 | |
| 8th week | Percentage of Repigmentation* | 6.00 ± 9.67c,d | 15.41 ± 11.57c | 13.88 ± 10.92d | 9.72 ± 7.32 |
| Score of Repigmentation* | 0.46 ± 0.63e | 0.91 ± 0.66 | 0.94 ± 0.63e | 0.66 ± 0.48 | |
| 12th week | Percentage of Repigmentation* | 17.00 ± 13.86f | 26.25 ± 12.08g | 26.94 ± 11.89f,h | 17.77 ± 8.78g,h |
| Score of Repigmentation* | 0.93 ± 0.70ı,j | 1.50 ± 0.67ı,k | 1.33 ± 0.59j | 1.00 ± 0.48k | |
| 24th week | Percentage of Repigmentation* | 30.00 ± 14.01l | 51.66 ± 12.30l,m | 54.44 ± 13.04n | 36.11 ± 14.90m,n |
| Score of Repigmentation* | 1.66 ± 0.72o | 2.41 ± 0.51o,p | 2.61 ± 0.50q | 1.77 ± 0.73p,q |
Table 4: Weekly treatment responses of patients with generalized vitiligo lesions.
*(Mean ± SD)
a: p=0.01, methylprednisolone aceponate vs pimecrolimus
b: p=0.009, methylprednisolone aceponate vs pimecrolimus
c: p=0.03, methylprednisolone aceponate vs pimecrolimus
d: p=0.03, narrow-band UVB vs pimecrolimus
e: p=0.04, narrow-band UVB vs pimecrolimus
f: p=0.03, narrow-band UVB vs pimecrolimus
g: p=0.03, methylprednisolone aceponate vs tacrolimus
h: p=0.01, narrow-band UVB vs tacrolimus
ı: p=0.04, methylprednisolone aceponate vs pimecrolimus
j: p<0.00, narrow-band UVB vs pimecrolimus
k: p=0.02, methylprednisolone aceponate vs tacrolimus
l: p<0.001, methylprednisolone aceponate vs pimecrolimus
m: p=0.006, methylprednisolone aceponate vs tacrolimus
n: p<0.001, narrow-band UVB vs tacrolimus
o: p=0.006, methylprednisolone aceponate vs pimecrolimus
p: p=0.01, methylprednisolone aceponate vs tacrolimus
q: p<0.001, narrow-band UVB vs tacrolimus
A comparison of the treatment groups in terms of the development of side effects revealed that the side effects were most commonly found in the NB-UVB group at weeks 2 and 12. The frequency of erythema, burning sensation and pruritus was significantly higher in the NBUVB group compared with the pimecrolimus and methylprednisolone aceponate groups at second week. (p<0.001, p<0.001, p=0.007, p<0.001, p<0.001, p=0.01, respectively) (Table 5).
| Methylprednisolone aceponategroup | Pimecrolimus group | Tacrolimus group | Narrow-band UVB group | ||
| 2nd week | Erythema* | 0.36 ± 0.49a | 0.35 ± 0.48b | 0.76 ± 0.83 | 1.15 ± 0.58a,b |
| Burning* | 0.68 ± 0.67c | 0.40 ± 0.68d | 0.47 ± 0.51e | 1.10 ± 0.44c,d,e | |
| Pruritus* | 0.63 ± 0.68f | 0.50 ± 0.82g | 0.57 ± 0.67h | 1.12 ± 0.45f,g,h | |
| 12th week | Erythema* | 0.15 ± 0.50ı,j | 0.80 ± 0.83ı | 0.38 ± 0.49 | 0.70 ± 0.65j |
| Burning* | 0.21 ± 0.41k | 0.25 ± 0.44l | 0.14 ± 0.47m | 0.85 ± 0.48k,l,m | |
| Pruritus* | 0.26 ± 0.45n | 0.25 ± 0.55o | 0.14 ± 0.35p | 0.75 ± 0.44n,o,p | |
| 24th week | Erythema* | 0.10 ± 0.31 | 0.15 ± 0.37 | 0.09 ± 0.30 | 0.05 ± 0.22 |
| Burning* | 0.31 ± 0.47 | 0.21 ± 0.41 | 0.89 ± 0.29 | 0.30 ± 0.47 | |
| Pruritus* | 0.31 ± 0.47 | 0.36 ± 0.49 | 0.14 ± 0.35 | 0.35 ± 0.48 |
Table 5: Development of side effects in groups in weeks 2nd, 12th and 24th.
*(Mean ± SD)
a: p<0.001, narrow-band UVB vs methylprednisolone aceponate
b: p<0.001, narrow-band UVB vs pimecrolimus
c: p=0.02, narrow-band UVB vs methylprednisolone aceponate
d: p<0.001, narrow-band UVB vs pimecrolimus
e: p<0.001, narrow-band UVB vs tacrolimus
f: p=0.01, narrow-band UVB vs methylprednisolone aceponate
g: p=0.007, narrow-band UVB vs pimecrolimus
h: p=0.006, narrow-band UVB vs tacrolimus
ı: p=0.006, pimecrolimus vs methylprednisolone aceponate
j: p=0.006, narrow-band UVB vs methylprednisolone aceponate
k: p<0.001, narrow-band UVB vs methylprednisolone aceponate
l: p<0.001, narrow-band UVB vs pimecrolimus
m: p<0.001, narrow-band UVB vs tacrolimus
n: p=0.002, narrow-band UVB vs methylprednisolone aceponate
o: p=0.003, narrow-band UVB vs pimecrolimus
p: p<0.001, narrow-band UVB vs tacrolimus
One patient each the methylprednisolone aceponate and pimecrolimus groups developed folliculitis. All adverse events resolved without sequelae, and neither atrophy nor telangiectasia was recorded.
Several treatment alternatives including topical corticosteroids, topical calcipotriol, topical calcineurin inhibitors and phototherapy (PUVA, UVB and NB-UVB) are employed either individually or in combination in the treatment of vitiligo. Despite this variety of treatment alternatives, the responses to treatment vary widely [3-7]. It is well known that face and neck lesions may respond better whereas acral lesions show resistance to treatment even when both lesion types occur in the same patient [11]. In the present study, we compared and contrasted the efficacy and side effects of four of the most commonly used alternatives in vitiligo treatment, which are topical pimecrolimus, tacrolimus, methylprednisolone aceponate, and NB-UVB.
NB-UVB treatment was carried out for vitiligo for a long time [12-14]. Kanwar, et al. [15] reported total repigmentation in 71.4% of vitiligo patients and mild to moderate repigmentation in 14.3% of the patients after one year of treatment with NB-UVB. Treatment response was found to be negatively correlated with disease duration in the present study, in which total repigmentation was achieved in the patients whose mean disease duration was 15 months, but only mild to moderate repigmentation developed in those who had the disease for a mean of 96 months. Better treatment response was achieved in the face and neck whereas the response was minimal on the hands, feet, knees and elbows, where there are acral and bone protrusions.
Brazzelli, et al. [16] reported that they achieved a satisfactory response to phototherapy in 80% of the 10 pediatric patients who were included in that study and that the side effects were limited and transient. In the present study, no significant correlation was established between the degree of repigmentation and the variables of skin type, positive family history and the distribution of the disease. As in other studies [13,14] the best treatment response was obtained in the face and neck area in our study. In addition, it has been reported that the patients with shorter disease duration responded better to treatment, and the need to start NB-UVB treatment as soon as possible in pediatric vitiligo patients was emphasized.
We also found that NB-UVB treatment was effective and reliable for vitiligo patients. Although no improvement was observed at weeks 2 and 4 with NB-UVB treatment, the highest repigmentation percentages and scores were obtained at weeks 8, 12, and 24. Among the vitiligo patients with acral involvement, the highest rates of improvement, relative to other methods, at weeks 2, 4, 8, 12, and 24 were observed in the patients receiving NB-UVB treatment. When compared in terms of the development of side effects, the most common side effects (erythema, burning sensation and itching) were observed in the NBUVB group at weeks 2 and 12. Because these side effects were welltolerated by the patients, it was concluded that NB-UVB treatment was effective and reliable in vitiligo.
Topical immunomodulators, such as tacrolimus and pimecrolimus, represent a novel therapeutic approach in the treatment of vitiligo, and they offer many advantages over corticosteroids for the management of chronic skin disorders in which prolonged treatment periods are needed [17,18].
The efficacy of pimecrolimus in the treatment of vitiligo is still controversial. Choi, et al. [19] compared the treatment efficacy in 52 vitiligo patients who was administered an immunomodulator treatment (51 tacrolimus and 1 pimecrolimus) for 6 months and 27 vitiligo patients who received topical steroid treatment. They reported that repigmentation started in a statistically shorter period of time in the topical immunomodulator group, but the outcomes of both treatments were similar, and topical immunomodulators were only as effective and reliable as topical steroids. There are other studies that report similar results [3,20,21]. Kose, et al. [22] used mometasone cream and pimecrolimus cream for 3 months in 40 pediatric patients with vitiligo. Although the mean rate of repigmentation was found to be higher in the patients using mometasone cream (65%) relative to those using pimecrolimus cream (42%), the difference was not statistically significant. However, that study concluded that mometasone cream was more effective on the body lesions whereas pimecrolimus was more effective on the facial lesions but not on others. In contrast to this study, Ho, et al. [23] established that pimecrolimus was as effective as clobetasol propionate in facial and non-facial lesions. The concerned study registered 100 pediatric patients with vitiligo between the ages of 2 and 16 and compared three different treatments (0.1% tacrolimus, 0.05% clobetasol propionate and placebo). Moreover, the patients were divided into two groups (those with facial lesions and those with non-facial lesions) and were followed for a period of 6 months. The use of tacrolimus and clobetasol propionate were found to have similar efficacy in both the facial and non-facial groups, and a statistically significant improvement was observed in both groups compared with the placebo group.
In the present study, we found an early but minimal response at week 2 with methylprednisolone aceponate and tacrolimus treatment. The efficacy of methylprednisolone aceponate was higher than those of the other treatment modalities at week 4. Methylprednisolone aceponate treatment was more effective than pimecrolimus and tacrolimus treatments at weeks 8 and 24.
Among the vitiligo patients with generalized involvement, the best treatment responses were achieved in the methylprednisolone aceponate group at weeks 12 and 24. However, in contrast to some previous publications, we found that methylprednisolone aceponate treatment was not more effective that pimecrolimus and tacrolimus treatments in patients with acral involvement. We did not establish a significant difference between tacrolimus and pimecrolimus treatment modalities in vitiligo patients in general and between those with acral and those with generalized involvement throughout the treatment period.
We found that methylprednisolone aceponate produced a prompt treatment response but that both methylprednisolone aceponate and NB-UVB treatment had the same efficacy over the long-term and produced better responses than pimecrolimus and tacrolimus. We did not find any side effects that were serious enough to require discontinuation of the treatment in any of the four groups.
In conclusion, several treatment alternatives are used either individually or in combination in the treatment of vitiligo. Despite the abundance of alternatives, there is a great variation in the patient responses to treatment. Therefore, there is still no consensus on the optimal treatment approach. We think that our study resolves some of the questions on this matter and should help guide clinicians in their choices of vitiligo treatment.
No conflict of interest
Download Provisional PDF Here
Aritcle Type: RESEARCH ARTICLE
Citation: Dertlioğlu SB (2018) Comparison of the Efficacy of Topical Tacrolimus, Pimecrolimus, Methylprednisolone Aceponate and Narrow Band UVB in the Treatment of Vitiligo. J Clin Cosmet Dermatol 2(3): dx.doi.org/10.16966/2576-2826.134
Copyright: © 2018 Dertlioğlu SB. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Publication history:
All Sci Forschen Journals are Open Access