Abstract
CCND1 and c-myc gene rearrangements are observed as a genetic change in the blastoid variant of mantle cell lymphoma, which is a rare subtype of double-hit lymphoma. Herein, we reported one case of nasal blastoid variant of mantle cell lymphoma with both CCND1/IGH and c-myc gene rearrangements in a 52-year-old man who had a solid mass in right nasal cavity. Histologically, the tumor was composed of pleomorphic medium to large-sized lymphoid cells with prominent nucleoli and active mitotic figures. Immunohistochemistry staining showed that the tumor cells were positive for vimentin, CD99, CD79a, Bcl-6, Bcl-2, CD5, cyclin D1, c-myc, SOX11 and MUM-1. Ki-67 expression was found in approximately 95% of tumor cells. Both CCND1/IGH and c-myc gene rearrangements were found in this case by fluorescence in situ hybridization. The differential diagnosis includes carcinoma, malignant melanoma, olfactory neuroblastoma, and rhabdomyosarcoma.
Keywords:
Mantle cell lymphoma; Blastoid variant; CCND1/IGH and c-myc gene rearrangement; Nasal cavity
Introduction
Lymphoma is not common in the paranasal sinuses or the nasal cavity. The most common type was diffuse large B-cell lymphoma, followed by extranodal natural killer/T-cell lymphoma, nasal type in adult [1-3]. Mantle cell lymphoma (MCL) is an aggressive mature B-cell lymphoma with t (11; 14) (q13; 32) translocation involving the CCND1 and immunoglobulin heavy chain (IGH) genes and very rare in nasal cavity and paranasal sinus [4-8]. Herein, we first reported a rare case of blastoid variant of MCL occurring in nasal cavity with both CCND1/IGH and c-myc gene rearrangements.
Materials and Methods
Biopsy tissues were fixed in 10% neutral buffered formalin, and sections were embedded in paraffin blocks. They were cut into 4 µm-thick sections and stained with hematoxylin-eosin staining. Immunohistochemistry staining was carried out using an EnVision kit (Dako, Carpinteria, CA). The following primary antibodies purchased from Dako Corporation were used: pan-CK, vimentin, CD99, CD79a, CD5, CD3, CD10, Bcl- 6, Bcl-2, c-myc, SOX11, cyclinD1, MUM-1, HMB-45, Melan-A, CD56, synaptophysin (Syn), chromogranin-A (CgA), S-100 protein, and Ki- 67. Positive and negative control slides were used. Fluorescence in situ hybridization (FISH) probes and IGH/CCND1 Dual Color, Dual Fusion Translocation Probe and c-myc Break Apart Rearrangement Probe was purchased from Gene Company limited (HK, China). In situ hybridization was performed according to the manufacturer’s instructions. Hybridization signals were analyzed using an Olympus BX51 microscope equipped with appropriate filters and images and captured using the CytoVision imaging system (Applied Imaging, Santa Clara, CA).
Results
Clinical findings
A 52-year-old man complained of nasal obstruction and intermittent rhinorrhea with blood for one week. Nasopharyngo-fiberoscope examination showed that an exophytic pinkish mass with smooth surface was observed in his right nasal cavity. The mass was fragile and bleed easily (Figure 1A). A computed tomography (CT) scan of the nose and paranasal sinuses demonstrated an irregular soft tissue mass filled in the right nasal cavity, ethmoid sinus and maxillary sinus with nasal septum deviation to the left. The lesion extended into the medial and posterior wall and resulted in bone destruction (Figure 1B). No mass was found in nasopharyngeal region. There was no lymph node enlargement or hepatosplenomegaly. Laboratory and bone marrow examination were normal.
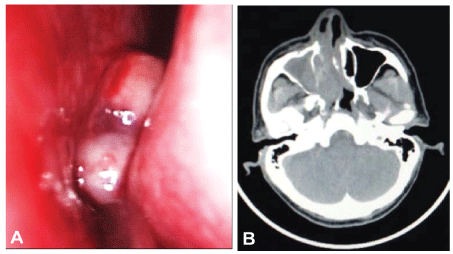
Figure 1: (A) Nasopharyngo-fiberoscope examination showed an exophytic pinkish mass with smooth outline in right nasal cavity; (B) CT scan demonstrated irregular soft tissue mass in the right nasal cavity, ethmoid sinus and maxillary sinus with nasal septum deviation to the left. The lesion extended into the medial and posterior wall and resulted in bone destruction
Pathological features
Grossly, the biopsy tissue was measuring 0.5 cm in the greatest diameter and gray-red. Microscopically, the medium to large-sized tumor cells with irregular nuclear and small to prominent nucleoli were arranged in diffused pattern with starry-sky appearance. Mitotic figures were about 15-20/10HPF. Atypical mitotic figures and focal necrosis were also found (Figure 2). Tumor cells were positive for vimentin, CD99, CD79a, Bcl-6, Bcl-2, CD5, cyclin D1, c-myc, SOX11 and MUM-1 by immunohistochemistry staining. Ki-67 expression was found in approximately 95% of tumor cells. Tumor cells were negative for CD10, CD3, CD56, HMB-45, Melan-A, Syn, CgA, CK, and S-100 protein (Figure 3). In addition, tumor cells were positive for both CCND1/IGH and c-myc gene rearrangements by FISH (Figure 4). Based on histological features, immunophenotype, and FISH findings, we made the final pathological diagnosis of nasal blastoid mantle cell lymphoma with both CCND1/IGH and c-myc gene rearrangements.
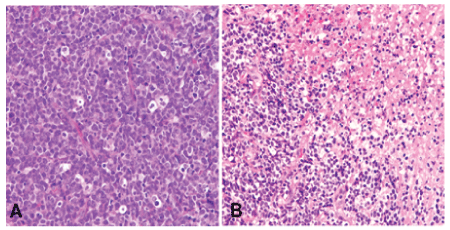
Figure 2: Histological features of nasal mass. (A) The tumor had a starry-sky appearance and was composed of medium to large-sized tumor cells with irregular nuclear and small to prominent nucleoli. Mitotic figures were easily identified, HE × 400; (B) Focal necrosis in the tumor, HE × 200
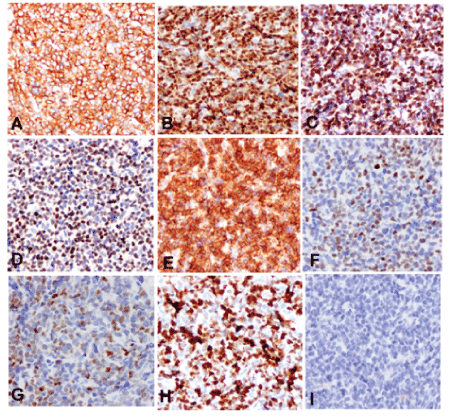
Figure 3: Immunophenotype of blastoid variant of mantle cell lymphoma. Tumor cells were positive for CD79a (A), cyclin D1 (B), SOX11 (C), c-myc (D), bcl-2 (E), bcl-6 (F), MUM-1 (G). Ki-67 expression was about 95% of tumor cells (H). Tumor cells were negative for CD10 (I) by immunohistochemistry staining, IHC × 400
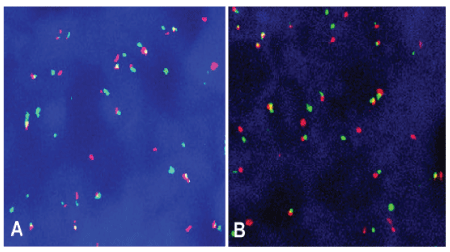
Figure 4: Tumor cells were positive for CCND1/IGH fusion signals (A) and c-MYC break-apart (B) rearrangement by fluorescence in situ hybridization (FISH), respectively. FISH × 400
Treatment and follow-up information
The patient received first 3 cycles of R-CHOP (rituximab, cyclophosphamine, hydroxyrubicin, vincristine, prednisone) and then 3 cycles of R-DHAP (rituximab, dexamethasone, cytarabine, cisplatin) chemotherapy. The tumor was complete remission after 3 cycles of R-CHOP and 2 cycles of R-DHAP chemotherapy. The patient was alive and well without tumor relapse at 7 months after diagnosis.
Discussion
MCL is a distinct type of B-cell non-Hodgkin’s lymphoma and typically composed of small to intermediate-sized lymphocytes. The tumor cells are immunoreactive for CD5, CD19, CD20, CD79, and surface immunoglobulin including IgM and IgD. The biological hallmark of MCL is the overexpression of cyclin D1 due to the t (11;14) (q13;q32) which is resulted in juxtaposition of the BCL1/CCND1 locus (11q13) and regulatory elements of immunoglobulin heavy chain genes (14q32) [4-6]. However, experimental data showed that overexpression of cyclin D1 is not sufficient for lymphomagenesis. Indeed, transgenic mice that overexpressed cyclin D1 developed B-cell lymphomas only when additional abnormalities were present [9]. Other chromosomal aberrations include bcl-2, bcl-6, and c-myc. Coexistence of CCND1 and c-myc gene rearrangements are observed as a genetic change in the blastoid variant of MCL, which has recently been classified with double-hit lymphomas [10-13]. To our knowledge, only 30 cases of double-hit lymphoma with CCND1 and c-myc genes rearrangement have been reported in English literature, most of which arose from lymph nodes [11-13]. Histologically, this type of lymphoma is composed of medium to large sized cells with prominent nucleoli and high proliferative activity. The patients are characterized by a poor prognosis and rapid relapse [11-14].
MCL is an extremely rare in nasal cavity and paranasal sinus. Only few sinonasal mantle cell lymphomas have been reported [1-3,8,15]. Herein, we first reported one case of nasal blastoid variant of mantle cell lymphoma with both CCND1/IGH and c-myc gene rearrangement. The differential diagnosis includes carcinoma, malignant melanoma, olfactory neuroblastoma, and rhabdomyosarcoma. Sinonasal carcinoma is typically arranged in sheets or nests. The tumor cells are positive for cytokeratin, and negative for lymphocyte markers. Malignant melanoma demonstrates solid, fascicular, epithelioid, spindled, plasmacytoid, pleomorphic, and rhabdoid morphologic features. Melanoma cells typically are positive for S-100 protein, HMB-45, and Melan-A, which are different from mantle cell lymphoma. Olfactory neuroblastoma usually grows in nests or in sheets. The tumor cells are uniform with small round nuclei, punctate chromatin, and small nucleoli. The histological feature is the presence of Flexner-type or Homer Wright–type rosettes. Olfactory neuroblastoma usually demonstrates diffuse positivity for neuron-specific enolase, synaptophysin, CD56, and Chromogranin A, which is different from mantle cell lymphoma. Rhabdomyosarcoma is composed of spindle or oval tumor cells with different degrees of rhabdomyoblastic differentiation and tumor cells are immunoreactive for myogenin and MyoD1 but not for lymphocyte markers, which is easy to differentiate from MCL.
Conflict of Interest
None declared.
Article Information
Article Type: Case Report
Citation: Li H, Zhen T, Shi H, Dong Y, Zhang F, et al. (2016) Nasal Blastoid Variant of Mantle Cell Lymphoma with Rearrangement of CCND1 and c-myc Genes with One Case Report. J Clin Case Stu 1(5): doi http://dx.doi.org/10.16966/2471-4925.131
Copyright: © 2016 Li H, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Publication history:
Received date: 03 Oct 2016
Accepted date: 15 Oct 2016
Published date: 20 Oct 2016





