Figure 1: Rotation plan for insecticides


Oscar Jaime Betancur Hurtado*
Elanco Animal Health, Colombia*Corresponding author: Oscar Jaime Betancur Hurtado Ph.D, Elanco Animal Health, Colombia, E-mail: betancur_oscar_jaime@elanco.com
Insecticides are a very useful tool when it comes to improving farm productivity and some of the public health indices [1]. It is well recognized that adequate vector control positively impacts productivity, reducing the presentation of diseases [2]. Arthropod vectors can transmit all kinds of pathogens, including viruses, bacteria, protozoa, helminths and rickettsiae [3]. The consideration of arthropods as vectors transmitting of pathogens that cause diseases is important both livestock and humans, given that some of the microorganisms have zoonotic potential, as in the case of Salmonella spp [4]. Some arthropods are recognized as pest and vectors on animal production, mainly house flies (Musca domestica), darkling beetles (Alphitobius diaperinus), poultry red mites (Dermanyssus gallinae), and cockroaches. In the most of cases, all of them are controlled through insecticides application.
Although insecticides are quite effective when used according to technical indications, some factors condition their effectiveness, such as the use of insecticides of poor quality or adulterated processes of resistance in insects, failures in the processes of preparation and application, among others [1]. For example, the application of sub dosed insecticides, or the exposure of insects to traces of these toxins, can generate greater tolerance in the future, and therefore the need to use a higher concentration than indicated to achieve its lethal effect [5].
Different pests have developed resistance due to mismanagement of the insecticides used [6]. Resistance can be understood as the inability of an insecticide used according to the technical indications to achieve an adequate control of a pest or group of pests, due to genetic modifications in the target species, which make them less sensitive to the applied product [7].The annual losses resulting from the resistance of pests to insecticides is more than $1.5 billion [5]. Resistance to pesticides is a problem that is increasing. It is estimated that more than 1000 pest species have resistance to at least one pesticide. Among the arthropods, the greatest resistance occurs in the order Diptera, followed by Lepidoptera, Coleoptera, Hemiptera and mites [8].
Among the more than 25 modes of action currently in the classification of IRAC (Insecticide Resistance Action Committee), 85% of these modes of action come from insecticides that act on the nervous and muscular systems. Of these, the group of neonicotinoids is the most representative on the market (27%), while the organophosphates, carbamates and pyrethroids together represent 31%. On the other hand, insecticides that modifies growth and development, as well as those that alter the production of energy (respiration), are 13%. At least 325 insecticides already have technical reports of resistance by one or more species [9]. Different levels of resistance have been found in the different classes of insecticides. DDT, organochlorines, carbamates, organophosphates, pyrethroids and pyrethrins are the chemical groups that present a higher level of resistance, largely due to their diversity of chemical compounds, and for the long time of use in the market; however, in other insecticides considered as new generation, resistance reports also have been found [10].
One of the ways to suspect resistance at field is the lack of efficacy of products applied at a known dose, which at other times allowed adequate control of the pest [11]. This is a reality that is observed every day in livestock production systems. Betancur and collaborators in 2016 carried out field work in Valle del Cauca, Colombia (unpublished data) to compare the efficacy of an organophosphate (chlorpyrifos) against a neonicotinoid (thiamethoxam) in a broiler farm; chlorpyrifos had been used for more than two years consecutively for the control of Alphitobius diaperinus and a lack of control over time had been perceived; after evaluating the effectiveness of each product in the poultry houses, it was found that thiamethoxam allowed a control >90% in both larvae and adults, while chlorpyrifos achieved a control <68%, considering these differences as statistically significant with p ≤ 0.05. Despite the fact that a laboratory study was not carried out to confirm the suspected resistance, it was clear that the use of the same insecticide for a long time should be avoided, to minimize the risks of resistance and achieve an adequate control of the pests. In this sense, the IRAC has mentioned that resistance should only be discussed when, in addition to the reduction of efficacy at field, a scientific test is made [10].
The mechanisms of resistance can be divided into two main groups: physiological and behavioral. Physiologically, the resistance can be presented by changes in the site of action of the insecticide, due to specific mutations in the receptors, which limits the ability of the toxic to bind to the receptor molecule, or affects its function after the union. Another mechanism is biotransformation, which involves the metabolic destruction of the insecticide within the organism, by means of biochemical processes such as hydrolysis, oxidation or conjugation [12], where some enzymes play a preponderant role (esterases, glutathione-S-transferases and cytochrome P450 monoxygenases) [13]. Resistance can also occur due to a reduction in the rate of cuticular penetration, which prevents the insecticide from reaching its lethal concentration, and on the contrary allows the body to metabolize and eliminate the drug with minimal or absent toxic effects. Finally, physiological resistance can occur due to the binding of the insecticide with non-objective macromolecules, so it does not exert any toxic action [12]. On the other hand, behavioral resistance should be understood as the heritable capacity of an insect to avoid contact with an insecticide, which results in a measurable decrease in susceptibility to a toxic [14].
The problem of resistance usually occurs exclusively on the active ingredient used, however, because different compounds of a particular chemical group share a common mechanism and site of action, the probability that cross-resistance is acquired with the other compounds from the same group is high [7]. Although cross-resistance is normally found among compounds with similar modes of action, some reports indicate that it is also possible that it occurs between pesticides that have different mechanisms of action [5].
The mechanisms of resistance in each group of insecticides are variable depending on the species of insect. In general, it is considered that the main mechanism of resistance in pyrethroids is genetic, and is known as knockdown resistance (kdr); it is a recessive allele that causes a mutation in the site of action of the insecticide [15,1]. In the case of organophosphates, the resistance mechanisms involved are mainly by over expression and potentiation of enzymes P-450 monoxygenases (P450s), glutathione S-transferases (GSTs), and hydrolytic enzymes (esterases, carboxylesterases), however, the molecular type resistance has been reported in several studies [16]. For neonicotinoids, it is considered that detoxification by monooxygenases is the main mechanism of resistance in some insects; this occurs as a consequence of over expression of genes related to enzymes such as P450s [17,18]. According to Sparks et al. [19] the most common resistance to spinosyns is produced by alteration of the site of action of the insecticide.
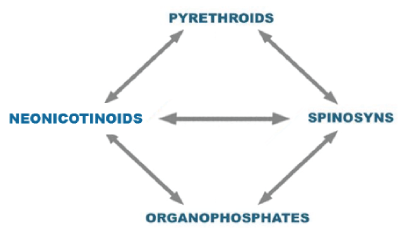
The best way to manage resistance to pesticides is to reduce the selection pressure of the toxic compound on the pest species, in other words, the complete elimination of susceptible pests by the pesticide should be avoided. To fulfill this purpose, it is necessary to use a comprehensive pest management program, avoid the unnecessary application of pesticides, use physical or biological control methods, and conserve areas free of chemical treatments where susceptible pests survive. However, in those situations where the use of pesticides becomes the only control tool, resistance management requires a rotation of the pesticides (Figure 1), which must be done between different chemical groups, and with different mechanisms of actions [8]. It is always recommended, to use products with proven effectiveness.

Figure 1: Rotation plan for insecticides
Figure 1 illustrates an example of a rotation plan for insecticides, considering some of the main classes of insecticides on the market. Some authors support it [1,15,17- 21]. For example, in broilers production to control Alphitobius diaperinus, rotation can be after 2-3 flocks of use. Other option is to apply neonicotinoids during spring/summer season, pyrethroids during summer/fall, spinosyns during fall/winter and organophosphates in winter/spring.
This scheme presents four groups of insecticides with differential mechanisms of action; pyrethroids act as modulators of sodium channels keeping them open, which produce hyper excitation, and sometimes nerve impulse blockage. For its part, organophosphates also cause hyper excitation by inhibiting the enzyme acetyl cholinesterase (AChE) (IRAC, 2017). As can be observed in figure 1, rotation between organophosphates and pyrethroids is not recommended, due to the possibility of cross-resistance between these two groups, possibly related to the enzymatic action of esterases [22], or monooxygenases [20]. Otherwise, the neonicotinoids exert their action as nicotinic acetylcholine receptor agonists postsynaptic (nAChR), and may cause manifestations such as hyper excitation, lethargy and paralysis [23]; its site of action reduces the possibility of cross-resistance with other insecticides such as pyrethroids and organophosphates [17]. Finally, the spinosyns, although acting on the nAChRs, do so in a different place than the neonicotinoids. In this way, spinosyns act with a double mode of action, by interacting at the same time with acetylcholine and GABA receptors at the synapsis level, which is a unique mechanism among the different classes of actives and offers to be less likely to develop resistances [24].
Regarding the monitoring of resistance, at present there are a diversity of methods that allow to know the existence of it; conventional methods include comparing insects collected in the field versus susceptible insects, by evaluating the LD50 of a given insecticide used at different dosages; another option is the exposure of the insects to a single dose of insecticide for a certain period of time, and assess their toxicity. The difficulty of these methods is that they do not allow obtaining accurate information about the resistance mechanism involved. For this reason, biochemical, molecular and immunological tests have been developed, which are very informative, and in addition, they are a key tool in the understanding of resistance processes, as well as the development of resistance management and prevention strategies [8].
Download Provisional PDF Here
Article Type: REVIEW ARTICLE
Citation: Betancur HOJ (2018) Insecticide Resistance Management: A Long Term Strategy to Ensure Effective Pest Control in the Future. J Anim Sci Res 2(1): dx.doi.org/10.16966/2576-6457.111
Copyright: © 2018 Betancur HOJ. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Publication history:
All Sci Forschen Journals are Open Access