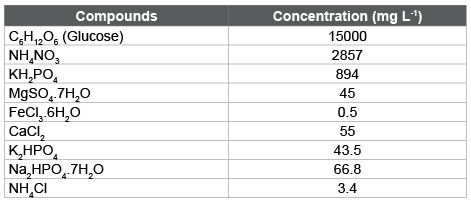
Table 1: Composition of synthetic wastewater

Barwal A Chaudhary R*
School of Energy and Environmental Studies, Faculty of Engineering Sciences, Devi Ahilya University, Takshashila Campus Khandwa Road, Indore, Madhya Pradesh, India*Corresponding author: Rubina Chaudhary (Reader), School of Energy and Environmental Studies, Faculty of Engineering Sciences, Devi Ahilya University, Takshashila Campus, Khandwa Road, Indore, Madhya Pradesh, India, Tel: +91731-2460309; Fax: +91731-2460737; E-mail:rubina_chaudhary@yahoo.com
Attempts were made in this study to examine the effectiveness of conventional chemical coagulation and flocculation process using ferrous sulphate, alum and Fenton process for the treatment of high chemical oxygen demand (COD) industrial wastewater. Removal of organic matter (expressed as COD) was investigated for highly organic wastewater having COD of 15000 mg/L. Also, the optimum conditions for coagulation/ flocculation process, such as coagulant dosage, Fenton dosage and pH of solution were investigated using jar-test experiment. The results revealed that in the range of pH tested, the optimal operating pH was 7.5-8 for FeSO4 and alum and 3 for Fenton process. Percentage removals of 26, 42 and 88 for COD was achieved by the addition of 1.0 g/L alum, 1.2 g/L of FeSO4 and 1:20 Fe2+/H2 O2 ratio, respectively. It can be concluded from this study that coagulation/flocculation may be a useful pre-treatment process for high organic load industrial wastewater prior to biological treatment.
Alum dosing; Coagulation; Chemical oxygen demand; Fenton reagent; Wastewater
In recent years, with an increase in the stringent water quality regulations due to environmental concerns, extensive research has focused on upgrading current water treatment technologies and developing more economical processes that can effectively deal with toxic and biologically organic contaminants in wastewater [1].
Industries manufacturing pharmaceuticals, cosmetics, organic dyestuff, soaps and detergents, pesticides and herbicides, tanneries and leather, paper, brewery and fermentation industry generate wastewater containing high organic load, toxicity or presence of bio-recalcitrant compounds having various origins and properties. Such wastewater having poor biodegradability needs a strong pretreatment method, followed by a biological treatment process [2]. Usually, conventional chemical coagulation - flocculation methods like Alum, ferrous sulphate, polyelectrolyte etc. are very commonly used as a pre-treatment method to enhance the bio-degradability of wastewater during the biological treatment. Among these chemical processes, the advanced oxidation process (AOP) has been efficiently used to reduce the organic load or toxicity of different wastewater [3]. Fenton reagent is considered as one of the AOP and used for the treatment of both organic and inorganic substances [4]. The Fenton’s reaction has a short reaction time among AOPs; therefore, it is used when a high COD removal is required [5].
Moreover, the reaction occurs at ambient temperature and pressure, involves safe and easy to handle reactants, no special equipment required, no mass transfer limitations, no energy involved and can be implemented with a great variety of compounds (6-8).
The Fenton’s system consists of ferrous salts combined with hydrogen peroxide (H2 O2 ) under acidic conditions. Ferrous ion reacts with hydrogen peroxide, producing hydroxyl radical ▪ OH mentioned below (reaction 1) [9-11].
H2 O2 + Fe2+ → Fe3+ + OH- + ° OH ……………. (1)
The ▪ OH free radical, having a very high oxidation potential (E°=2.80 V), is capable of reacting with many organic species through a series of chain reactions.
Fe3+ + H2 O2 → Fe2+ + HO2 • + H+ ……………. (2)
Fe3+ + HO2 • → Fe2+ + O2 + H+ ……………. (3)
Fe3+ + R• → Fe2+ + R+ ………….… (4)
Fe3+ produced can react with H2 O2 and hydroperoxyl radical in the so-called Fenton-like reaction, which leads to regenerating Fe2+ (reactions 2 and 3). Fe2+ regeneration is also possible by reacting with organic radical intermediates (Reaction 4) [12].
Fe3+ produced can react with H2 O2 and hydroperoxyl radical in the so-called Fenton-like reaction, which leads to regenerating Fe2+ (reactions 2 and3). Fe2+ regeneration is also possible by reacting with organic radical intermediates (Reaction 4) [12].
It was also reported by Ivan et al. [13] that ▪ OH reacts unselectively within a millisecond with organic substances. H2 O2 and Fe2+ also had a synergistic effect on the removal of colloidal organic residues by coagulation.
In Fenton treatment, the pH value should be near 2-4 during the reaction. After reactions are completed, precipitation of the oxidized iron as Fe(OH)3 occurs by neutralizing or adjusting the pH to 7.5 - 8 [14,1]. Neyens et al. and Neyens and Baeyensin [15,11] studied the effects of pH, temperature, reaction time and H2 O2 concentration with considerable reduction inorganic concentration.
As per the literature review, no study has been performed for wastewater having extremely high COD value. Therefore, the objective of present study was to evaluate the efficiency of conventional coagulants and Fenton’s reagent to remove COD from industrial wastewater characterized by its extremely high value of COD (approximately 15000 mg/L) and a low value of BOD, probably due to the presence of toxic compounds, which hamper a direct biological treatment and thus require a chemical pre-treatment. Effects of pH and the optimal dosages of conventional coagulants and Fenton reagent (Fe2+/H2 O2 ) were also determined.
Synthetic industrial wastewater was prepared in the laboratory with tap water by mixing different chemicals containing organic carbon, macro and micro-nutrients. The composition of stock synthetic wastewater was adjusted in such a way that COD becomes approximately about 15000 mg/L. The working synthetic wastewater containing varying COD concentrations was prepared by diluting appropriate volume of stock synthetic wastewater with tap water. The composition of synthetic wastewater is mentioned in table 1.
The coagulation, flocculation and Fenton system experiments were conducted using Jar Test equipment (Make- Jindal SM Scientific Instruments Pvt. Ltd., India), consisting of 6 jars of 1000 mL each whose contents were stirred with flat stirring paddles (25 mm × 75 mm) as shown in the figure 1.
Analytical procedures were monitored in accordance with standard methods [16]: COD with different coagulants. pH was measured by using a digital pH-meter. COD removal efficiency (RE) was calculated by using the following equation 1:
$$RE\left( \% \right)\, = \left( {{{{C_{in}}\, - {C_{eff}}} \over {{C_{in}}}}} \right)\, \times \,100\,..................\left( 1 \right)$$
Where Cin and Ceff are the concentrations in the influent and in the effluent, respectively.
All chemicals used in this study were analytical grade and obtained from Merck. Hydrated lime (Ca(OH)2 ) was used in flocculation step was purchased as commercial. Distilled water was used to prepare the desired concentration of solutions. FeSO4 , and alum (KAl(SO4 )2 •12H2 O) were used as conventional coagulants.
For each jar test, rapid mixing conditions were 1 min at 120 rpm and slow mixing conditions were 20 min at 30 rpm in order to favor flocs aggregation. Then, the samples were allowed to settle for 30 min. After the settling period, volumes of about 10-20 mL were taken 2 cm below the surface level using a plastic syringe. For the purpose of coagulation, pH was adjusted with the lime dosing. A series of three Jar-test experiments was conducted with different coagulants with variable dosing while maintaining the optimum pH. Each jar test series was conducted in duplicate. In the first and second jar-test experiment, conventional coagulants like alum and ferrous sulfate doses were added at variable dosing of 250, 500, 700, 1000, 1200 and 1500 mg/L respectively.

Table 1: Composition of synthetic wastewater
Figure 1: Experimental setup
Third jar-test was performed with Fenton process (Fe2+/H2 O2 ). This process serves both oxidation and coagulation functions. The pH level has to be decreased to 2-3 for the effective chemical oxidation and flocculation of the complex organic materials dissolved in water. The pH level (2.0, 2.5, 3.0 and 3.5) of the wastewater was set by H2 SO4 (6N). After the setting of the pH level, desirable amounts (Fe2+/H2 O2 ratio) of FeSO4 •7H2 O solution were added to the reaction solution as the ferrous iron (Fe2+) source. The reaction was assumed to start with the addition of H2 O2 (ranging from 250 to 2500 mg/L). Initially the reaction time was set as 0.5 h. Later, it was extended up to 2 h. After the selected reaction time, the experiment was ceased with the addition of lime as to increase the pH to around 8, to precipitate ferrous iron out as solid Fe(OH)3 . Residual COD of the supernatant was measured after settling for 30 min.
Conventional chemical oxidation-coagulation consists of the direct dosing of coagulants to the wastewater to reduce the electrical repulsion forces that inhibit the aggregation of particles. Iron (FeSO4 /FeCl3 ) and aluminum (alum) have been widely used as coagulants according to the literature, because they are cheap and have been demonstrated to be very effective on the coagulation process [17]. In Jar test-I and II, conventional coagulants like alum and ferrous sulfate were used at different doses of 250, 500, 700, 1000, 1200 and 1500 mg/L respectively. Simultaneously, lime dosing was added in the wastewater sample to maintain the pH value near 7.0 - 7.5. Figures 2 and 3 represent the COD reduction values which were obtained after conducting the test. It was observed that maximum COD reduction efficiency was 26 and 42% at the dosing of 1200 mg/L of alum and 1000 mg/L of FeSO4 respectively for each jar test.
In Fenton process, there are various factors which affect the COD removal efficiency.
Effect of H2 O2 dosing: Parameters affecting the Fenton process include operating pH and dosages of FeSO4 and H2 O2 . Operating pH of the system has been observed to significantly affect the degradation of pollutants (18-22, 4). The oxidation potential of hydroxyl radicals (▪ OH) is known to decrease with an increase in the pH [23,3]. In this study, optimum dose of H2 O2 was determined first. The experiments were performed in the wide range of 250 - 2500 mg H2 O2 /L. As the dosage of H2 O2 changed from 250 to 1500 mg/L, COD removal efficiency increased from 16.2 to 73.5% (COD value 12385.6 and 3916.7 mg O2 /L respectively). However, above the dose of 1500 mg H2 O2 /L a slight decrease in COD removal was observed (Figure 4). This could be explained by the excess of hydrogen peroxide concentration, behaving as a hydroxyl radical’s scavenger in the Fenton reaction. This finding is in line with the reports of Lin et al. [18], Kang and Hwang [19] Ozdemir et al. [4].
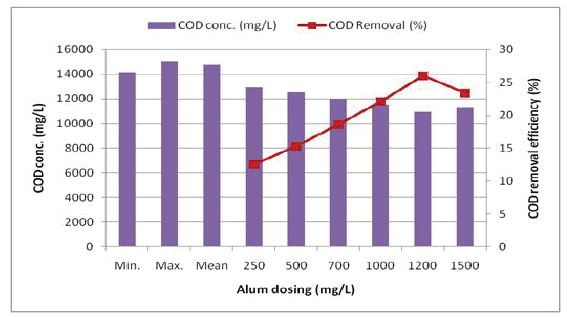
Figure 2: COD removal efficiency for alum treatment in the coagulationflocculation process
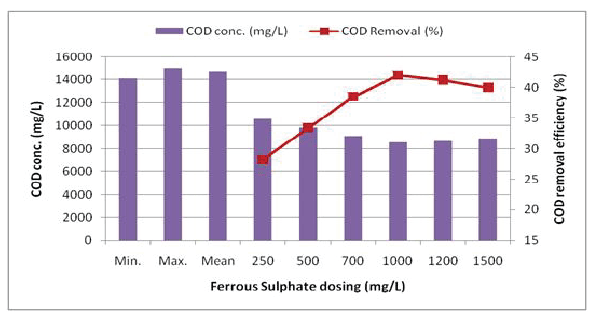
Figure 3: COD removal efficiency for ferrous sulfate treatment in the coagulation-flocculation
Effect of pH: pH has been observed to significantly affect degradation of COD. The pH of the system was varied from 2.0-3.5 and the best removal efficiency was observed at pH 3.0 (Figure 5). At pH<3.0, there was reduction in the removal efficiency of COD. This may be due to the formation of Fe (II) complex which reacts more slowly with H2 O2 produces fewer amount of °OH radicals thereby reducing the removal efficiency. Beyond pH>3.0, the COD removal efficiency also reduced due to decrease in the free Fe ions due to the formation of Fe (II) complex.
Effect of Fenton (Fe2+/H2 O2 ) dosing: To investigate the optimum FeSO4 dosage, five different Fe2+/H2 O2 ratios (1:5, 1:10, 1:15, 1:20 and 1:25) were tested using optimized H2 O2 concentration of 1500 mg/L (Figure 6). These results show that with an increase in Fe2+/H2 O2 ratio from 1:5-1:20, COD removal efficiency significantly increases from 53% to 88%. However, above Fe2+/H2 O2 ratio of 1:20, only slight changes in COD removal were observed and COD concentration kept almost the same level about 6946.6-1773.6 mg/L. Therefore, optimum ratio of Fe2+/H2 O2 was observed to be 1:20.
Effect of reaction time for Fenton reagent: Reaction time for Fenton reagent is also considered as an important factor. The reaction time was extended to 2 h. The results demonstrated that COD removal efficiency was rapidly increasing within the first 30-45 minutes of reaction time. Maximum efficiency of COD removal (62.9%) was observed after 60 min. After this time, COD removal was diminished to 61.4% and 52.3% after 90 and 120 min. respectively (Figure 7). This could be explained by the formation of by-products more resistant to oxidation.
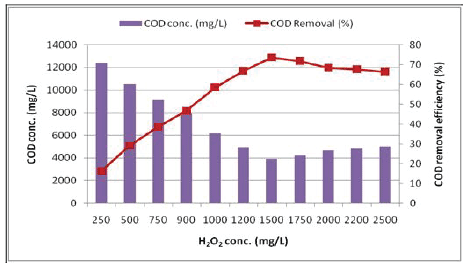
Figure 4: Effect of H2 O2 concentration on the COD removal efficiency
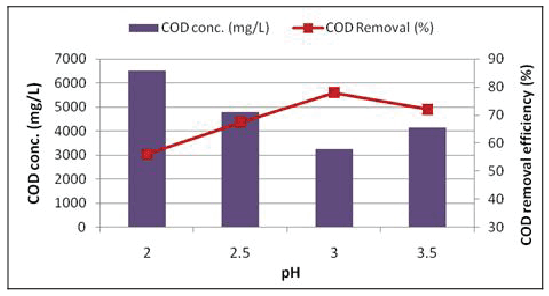
Figure 5: Effect of pH on the COD removal efficienc (H2O2 conc. = 1500 mg/L)
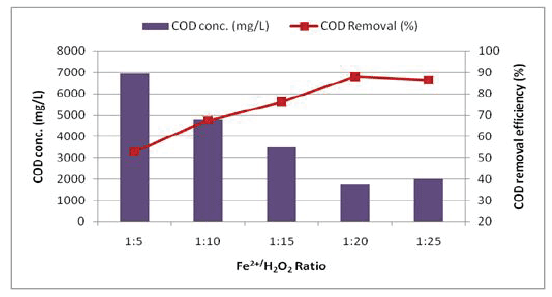
Figure 6: Effect of Fe2+/H2 O2 dosage on the COD removal efficiency (H2 O2 conc. = 1500 mg/L; pH = 3)
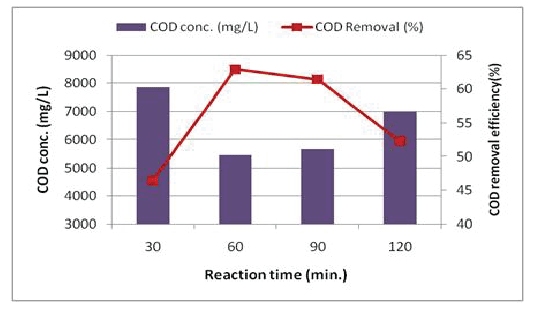
Figure 7: Effect of the reaction time on the COD removal efficiency (H2 O2 conc. = 1500 mg/L; Fe2+/H2 O2 ratio = 1:20; pH = 3).
The process of coagulation-flocculation applied to the highly organic wastewater was found effective in COD removal using H2 O2 and Fe2+. The best removal efficiency (88%) was obtained at pH 3.0 using a Fenton (Fe2+/H2 O2 ) ratio of 1:20 with H2 O2 doing of 1500 mg/L. The COD removal efficiency (62.9%) was found rapidly increasing at an optimum reaction time of 60 minutes. At acidic pH values, it has been shown that H2 O2 decomposes to produce ▪ OH radicals. When only conventional coagulants like alum and FeSO4 were used, the COD removal efficiency was only 26 and 42% respectively. It is obvious that Fe2+/H2 O2 + had a strong synergistic effect on coagulation and achieve the best degradation in terms of COD removal and appears to be useful in increasing the biodegradability of wastewater that contains complex compounds. But, from economical point of view, Fenton process has higher cost if compared to other coagulants but this cost could be compensated by lower consumption of disinfecting agents and the lower costs of sludge handling and disposal.
Download Provisional PDF Here
Article Type: Research Article
Citation: Barwal A, Chaudhary R (2016) Feasibility Study of Conventional Coagulants and Fenton Reagent for High Chemical Oxygen Demand Wastewater. Int J Water Wastewater Treat 2(2): doi http://dx.doi. org/10.16966/2381-5299.118
Copyright: © 2016 Barwal A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Publication history:
All Sci Forschen Journals are Open Access