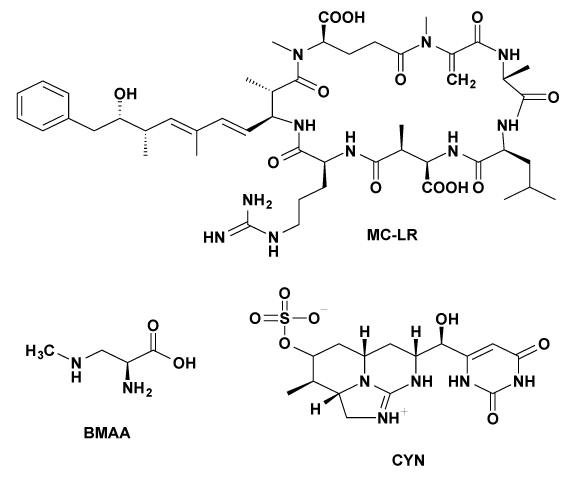
Figure 1: Molecular structures of the cyanotoxins microcystin-LR (MC‑LR), β-N-methylamino-l-alanine (BMAA) and cylindrospermopsin (CYN).

Evelyn Balsano1 Maranda Esterhuizen-Londt1 Enamul Hoque2 Stephan Pflugmacher1*
*1Technische Universität Berlin, Department of Ecotoxicological Impact Research and Ecotoxicology, Ernst-Reuter-Platz 1, 10587 Berlin, Germany*Corresponding author: Stephan Pflugmacher, Technische Universität Berlin, Department of Ecotoxicological Impact Research and Ecotoxicology, Ernst-Reuter-Platz 1, 10587 Berlin, Germany, Tel: +49 3031429023, Fax: +49 3031429022, E-mail: stephan.pflugmacher@tu-berlin.de
Cyanobacteria produce various harmful secondary metabolites, which pose a serious global threat to aquatic ecosystems and human health. Biodegradation is an important topic of water purification research and offers especially an environmentally friendly remediation strategy. Here, we present a water fungus of the genus Mucor that shows considerable promise to be applied as a mycoremediation agent for the removal of cyanobacterial toxins from aquatic environments. In the present study, we investigated the effect of three different cyanobacterial toxins, namely the hepatotoxin microcystin-LR, the neurotoxin β-N-methylamino-l-alanine and the cytotoxin cylindrospermopsin, on the sensitivity of Mucor hiemalis EH5 using an adaptation of the Kirby-Bauer disk diffusion assay, and the influence of the toxins on fungal growth and biomass production via radial extension and dry weight (DW) measurements. Additionally, we established an optimized strategy for the individual cyanobacterial toxin extraction from the vegetative part of Mucor hiemalis EH5 and analyzed its biosorption potential via LC-MS/MS measurements. The fungal microorganism showed a fast adaptation behavior and strong resistance towards the toxins. No significant differences in terms of growth were perceived when comparing the exposed fungi to an untreated control. This indicates that the cyanobacterial toxins are not lethal to the fungus and that the organism can grow and develop undisturbed in their presence. Toxin uptake was quantified by LC‑MS/MS detection with recoveries for the established extraction methods of >60-85%. After exposing the fungi to each of the toxins respectively for 24 and 48 hours, we found a significant uptake (p<0.05) in the range of 0.1 to 1.7 mg of the applied toxin per gram mycelial biomass (dw). Our results suggest that Mucor hiemalis EH5 is an ideal organism to be tested in further studies as a biodegrading system for the remediation of cyanobacterial toxins from contaminated waters.
Cyanobacterial toxins; Biodegradation; Mucor hiemalis EH5; Growth response; Biosorption; Toxin uptake
Cyanobacteria are ubiquitous organisms, mostly found in aquatic environments. They are amongst the earliest organisms on Earth and the pioneers of oxygen production. Even though their evolutionary and ecological importance remains uncontroversial [1], they are now a growing environmental and public health concern because of their ability to form various bioactive secondary metabolites [2,3]. Eutrophication of water bodies and climate change factors promote the development of microalgae and cyanobacteria yielding to an explosive formation of massive blooms [4,5]. The produced cyanobacterial toxins are mainly retained within the cyanobacterial cells but are especially released during senescence and cell lysis [6,2]. These secondary metabolites display diverse modes of action thereby manifesting adverse effects on the aquatic flora and fauna [7-10] and human health [11]. According to their biological effects, they can be divided into five different groups: hepatotoxins, neurotoxins, cytotoxins, dermatotoxins and lipopolysaccharides [2]. Their structural diversity is clearly illustrated in Figure 1, which shows three commonly occurring cyanotoxins.
Microcystins, cyclic heptapeptides, are the most widespread toxins and are therefore the best studied. Microcystin-LR (MC-LR, Figure 1) is considered the most toxic compound of this family [12]. β-N Methylamino-L-alanine (BMAA, Figure 1) is a highly reactive nonprotein amino acid likely synthesized by all cyanobacteria [13-15] and can occur either free or protein-bound [16]. BMAA is assumed to cause various neurodegenerative diseases such as ALS-PDC and Alzheimer’s disease [16] and recently has been shown to induce neural damage at very low concentrations [17,18]. Cylindrospermopsin (CYN, Figure 1) has caused human poisonings in Australia and Brazil [19] and is accountable for the death of animals [20].
Cyanobacterial toxin removal from water bodies is of critical importance [21] and an emerging research area of increasing interest in order to improve water quality and safety. Biosorption and biotransformation proves to be the most appropriate method for the efficient elimination of cyanobacterial toxins from water bodies in environmental conditions [22] and offers particularly the advantage of a natural and sustainable strategy [23].
Fungal biosorption is achieving prime attention in effluent treatment processes. Extensive research exists on metal biosorption by terrestrial and aquatic fungi as an alternative treatment for heavy metal bearing wastewaters. The sorption of heavy metals and radionuclides by fungi such as Aspergillus spp., Mucor spp., Rhizopus spp. and Penicillium spp., and yeast (Saccharomyces spp.) has been observed to varying extents [24-27].
Furthermore, fungal uptake and biodegradation of different xenobiotics is known. Four selected ectomycorrhizal fungi have been shown to almost completely remediate 1,1,1-trichloro-2,2-bis (4-chlorophenyl) ethane (DDT) from the media within 15 days and only 40-50% was found being accumulated in mycelia, whereas the remaining DDT was degraded to metabolites, which were identified by GC-MS [28].

Figure 1: Molecular structures of the cyanotoxins microcystin-LR (MC‑LR), β-N-methylamino-l-alanine (BMAA) and cylindrospermopsin (CYN).
The most successful application of white rot fungi lies in the purification of effluents of textile industries, paper and pulp industries, because of their high decolorization capacity. Fungal mycelium of Trametes veriscolor showed initial adsorption of synthetic dyes in the first hour of contact of the dye with the mycelium [29]. An efficient decolorization of 17 disperse dyes due to sorption of dyes to fungal cells was also observed in Cunninghamella polymorfa cultures [30]. In general, dye molecule biosorption onto cell surface appears to be quick and often completes in a few hours. Amino, carboxyl, thiol and phosphate groups present in the fungal cell wall are responsible for binding dye molecules [31]. Fu and Viraraghavan [32] studied biosorption of four dyes using Aspergillus niger biomass. Phanerochaete chrysosporium (P. chrysosporium) is used in the MyCoR (mycelial color removal) reactor [33] or immobilized on alginate beds it serves for the removal of chlorophenols [34].
To date, despite the important role of fungi in water treatment, very limited information exists on fungal biosorption and degradation of cyanotoxins. Research has primarily focused on screening bacteria for their ability to control and degrade harmful cyanobacteria and their produced toxins in aquatic environments. Little attention has been paid to fungi, including studies on white rot fungi, which can inhibit the growth of cyanobacteria species [35-37]. Moreover, the ability of the white rot fungus Trichaptum abietinum 1302BG to degrade MC-LR in a culture of Microcystis aeruginosa PCC 7806 has been reported. After 12 hours complete degradation of extracellular MC-LR was observed. More recently, the strain Trichoderma citrinoviride has been identified to selectively inhibit the growth of the cyanobacterium Microcystis aeruginosa and effectively degrade its microcystin toxins. After 72 hours complete elimination of the toxin was achieved [38]. These results show that fungal strains can degrade microcystins more rapidly (12-72 hours) than bacterial strains (6-25 days) [39,40]. Thus it is necessary to screen more fungal species, especially aquatic fungi, which may be used as efficient bio agents against cyanobacterial toxins.
Eukaryotic fungi exceed bacteria in their degradation ability due to their very low substrate specificity [41,42]. While bacteria work through genetic specific degradation mechanisms, fungi act with unspecific extracellular oxidation enzymes, such as the peroxidase systems [43,44] and the glutathione-S-transferase enzymes [45]. Glutathione-Stransferases are key enzymes in the detoxification metabolism and protect the cell against toxicants by catalyzing the conjugation of xenobiotics to glutathione. Fungal species with glutathione-S-transferase activity, like the genera Basidiomycotina, Deuteromycotina or Zygomycotina, e.g. Cephalosporium, Penicillium, Trichoderma and Mucor spp. can be utilized for the purification of sulfidic waters [46]. Mucor hiemalis (M. hiemalis) F. irnsingii (DSM 14200, alias EH5), which has been isolated from such waters, possesses a distinctive high glutathione-S-transferase activity and shows a high tolerance against H2 S [47]. Moreover, the fungus has functional groups on the cell wall enabling biosorption of heavy metals, such as chrome VI [48] and nickel [49], and is also known for its fast and complete remission potential of the herbicide isoproturon, if it is used in combination with P. chrysosporium [47]. However, P. chrysosporium requires an optimal growing temperature of 39° C leading to high temperature expenditure in biotechnological applications. In contrast, M. hiemalis EH5 is temperature-independent, and still sporulates at temperatures lower than groundwater temperature (e.g. 5° C). This paves the way for a season-independent application of the fungal organism and enables pollutant removal in deep sediment layers as well as under extreme environmental conditions. M. hiemalis can be applied in facultative aerobic/anaerobic, reducing or H2 S polluted water; moreover it resists metal contamination over a wide pH range (3-11) and can be used in ground and surface water, sewage, wastewater, and industrial and mine waters [50].
Because of the promising characteristics of M. hiemalis EH5 to possibly degrade and remove cyanobacterial toxins from contaminated water bodies, the goal of the present work was to obtain an insight into the effect of MC-LR, BMAA and CYN on the sensitivity and growth responses of M. hiemalis EH5 and its biosorption ability towards the cyanotoxins.
M. hiemalis EH5 (DSM 14200), previously isolated as an aquatic H2 S- resistant strain from the sulfidic- sulfurous Irnsing spring water biofilms in Bavaria, Germany [45], was obtained from the culture collection of the Leibniz Institute DSMZ – German Collection of Microorganisms and Cell Cultures.
The cyanobacterial toxins microcystin-LR(MC-LR), β-N-methylamino-L-alanine hydrochloride (BMAA) and cylindrospermopsin (CYN) were purchased from commercial suppliers (Enzo Life Sciences, Alexis Biochemicals ALX-350-012-M001; Sigma-Aldrich, Munich, Germany CAS Number: 16676-91-8; Enzo Life Sciences, Alexis Biochemicals ALX-350-149-M001). Pre-stock solutions with a concentration of 1 mg/mL were prepared in pure methanol and stored at -20° C. Serial dilutions (5, 50, 100, 250, 500 and 1000 µg/L) were prepared in sterilized double distilled water for the experiments.
Short-term conservation of the pure culture in the laboratory was achieved by periodical inoculation of malt extract agar plates once per month.
For strain maintenance and propagation, cultures were grown on a solid malt extract agar substrate. The nutrient medium consisted of 30 g malt extract broth (Sigma-Aldrich, Fluka 70146), 15 g agar bacteriological (Oxoid no 1, LP0011) in 1 L double distilled water (Roth, T172.3) supplemented with 0.82 mM sodium thiosulfate and 100 ppm streptomycin sulfate (Roth, HP66.1) [45]. The medium was autoclaved for 20 min at 121° C. After cooling to 60° C, 10 mL of the agar medium were aseptically poured into petri dishes. The plates were sealed with parafilm and stored in the dark at 4° C for further experiments.
Mycelial growth experiments were assessed in the dark at 25° C, which has been reported to be the optimum growing temperature for M. hiemalis EH5 [51]. A mycelial mat containing sporangiospores of a 3-4 week old colony was gently removed with a sterile pair of tweezers from a 1 cm2 agar surface and positioned at 1 cm distance to the petri dish wall.
The procedure was adapted according to a modification of the Kirby-Bauer disc diffusion test [52]. Small filter paper disks, with an approximately diameter of 5 mm, were immersed in a highly concentrated toxin solution (100 μg/mL) of MC-LR, BMAA and CYN respectively. Different disks were used per toxin and placed onto separate agar plates for the inhibition zone assay studies. Water was used as a negative control, and antimitotic (±)-miconazole nitrate salt as a positive control. Zone diameters were measured from edge to edge across the zone of inhibition over the center of the disc. The zone of inhibition of fungal growth is used as a measure of susceptibility. Large zones of inhibition indicate that the organism is susceptible (S), while small or no zones of inhibition indicate resistance (R). Three independent replicates were performed.
Agar plates separately coated with 1 mL of MC-LR, BMAA and CYN toxin solution at different concentrations (5, 100, 250, 500 and 1000 μg/L) on malt extract agar were prepared. Five mm diameter mycelial disks were taken from a 3-4 week old grown culture and transferred onto the plates. The plates were incubated at 25° C in the dark.
Radial extension was marked at intervals of 24 hours for a seven days period or until the maximum extension was reached. Colony diameters were measured starting from the center of the inoculum. Distance values were expressed as the average of three measurement points of the plate (one middle axis and the left and right axes at an angle of 45°). The radial growth rate was calculated by linear regression of the colony radius versus time.
For biomass production evaluation, the dry weights were determined. The fungal colony was lifted out of the plate and transferred with the agar gel into 50 mL tubes filled with distilled water. The tubes were heated up to 110° C for 30 min in the autoclave in order to melt the agar, the content was then immediately poured through a strainer with a mesh size of 0.5 mm and rinsed with distilled water. Intact mycelia were collected on pre-weighted filter papers (Whatman no.1 pore size), dried in the oven at 80° C until a constant mass was reached (about 20 hours) and cooled to room temperature in a desiccator. Dry weights were noted after seven days of incubation [53].
Biosorption: exposure scenario and sample collection: Ten days old cultures of M. hiemalis EH5 grown on Sabouraud Dextrose Broth (Sigma-Aldrich, Fluka S3306) agar medium in petri dishes at 25° C in the dark, were exposed to 1 mL of a 1000 μg/L concentrated MC-LR, BMAA and CYN solution separately for 24 and 48 hours under optimal growth conditions. Five replicates were performed and pure water was used for the untreated controls. Before harvesting, fungal mycelia were washed thoroughly three times with five mL water, followed by one washing step with five mL methanol and repetitively three times with five mL water to completely remove all toxin residues from the plate and the mycelial mat surface. Then, mycelia were collected with a pair of tweezers, snap frozen in liquid nitrogen and lyophilized overnight (-48.3° C, 0.1163 mbar).
Toxin extraction: Disruption and homogenization of the vegetative part of M. hiemalis EH5 was achieved by Ultra-Turrax treatment (25,000 rpm, for max. 30 s) followed by glass potter grinding of the lyophilized mycelia (20-50 mg dw) in 1-1.5 mL of the respective disruption/extraction solvent described for each toxin below in this section. As sporangiospores were resistant to the mechanical disruption method used, only the toxin content in the mycelia of the fungi could be determined.
MC-LR was extracted with 0.1% trifluoroacetic acid (TFA) in 70% methanol. Each mycelial homogenate was sonicated for two hours in a water bath, shaken for 45 min and centrifuged (4000 xg for 10 min). The supernatant was collected and the pellet re-suspended in 500 μL of the extraction solvent. The shaking and centrifuging cycle was performed three times in total. The combined extracts were evaporated to dryness at 30° C in a vacuum concentrator and the obtained dried fractions were re-dissolved in 250 μL methanol 100% (MS grade) and centrifuged before insertion to the HPLC-MS/MS system.
BMAA was extracted following the protocol applied for BMAA extraction from cyanobacterial isolates [54] by sonication with 0.1 M trichloroacetic acid (TCA) for one hour in a water bath. Free BMAA was obtained in the supernatant after centrifugation at 15,800 xg for 3 min at 4° C to precipitate proteins. The pellet was washed twice with 250 μL of 0.1 M TCA, and all supernatants were combined. In order to release protein-bound BMAA, the pellet was suspended in 1 mL 6 M hydrochloric acid and hydrolyzed overnight. BMAA was derivatized prior to HPLCMS/MS analysis using the Phenomonex EZ:faastTM kit following the manufacturer’s specifications. The derivatization involves a concentration step on a sorbent tip, washing, elution from and removal of the stationary phase, and derivatization with a proprietary chloroformate derivative as well as sample clean-up via liquid-liquid extraction and evaporation of the organic solvent under a gentle stream of nitrogen. The remaining dried amino acid derivatives were re-dissolved in a mixture of LC mobile phase components (water/methanol, 3.2:6.8).
A modification of the method published by Welker et al. [55] has been applied for CYN extraction and pure water has proven to efficiently extract CYN from mycelial material. The homogenized mycelial water suspension was sonicated for 15 min in a water bath, shaken for one hour, sonicated again and centrifuged for 15 min at 4000 xg. The combined supernatants were dried in a vacuum concentrator and the dried fractions re-suspended in a mixture of acetonitrile and water (95:5). All steps were performed in the dark, as CYN is light sensitive.
LC-MS/MS: The chromatographic separation of MC-LR was accomplished with a Kintex C18 column (2.6 μm, 2.1 x 50 mm) on an Agilent 1200 Infinity Series liquid chromatography system coupled to an Agilent Technologies 6460 Triple QTM. The column oven temperature was set to 40° C and the injection volume used was 10 μL. A flow rate of 0.2 mL/ min was used during the analysis, using a solvent gradient with 0.1% TFA in H2 O (MS grade, mobile phase A) and 0.1% TFA in acetonitrile (MS grade, mobile phase B) for separation. At the start of the run, mobile phase B was increased from 0 to 35% over 3 min followed by an increase to 65% until 3.75 min, abidance at this condition was held for 5 min, concluding with a post time of 3 minutes. The retention time of MC-LR was 6.15 min. For the subsequent MS–MS detection, the MRM mode (positive mode) was used with a mass transfer of 995.5 (Q1) and 135, 213 and 379 (Q3) for MC-LR. Method calibrations were linear (R2 = 0.999) between 0.01 and 100 μg/L, with a lower limit of quantification being 2 pg on column.
Derivatized BMAA and internal standards were chromatographically separated on a Phenomenex AAA-MS amino acid analysis column (2.0 x 250 mm, included in the kit) (on the same equipment) at a column oven temperature of 35° C. The sample injection volume was 1 µL at a flow rate of 0.25 mL/min using the following solvent gradient with 10 mM ammonium formate in H2 O (MS grade, mobile phase A) and 10 mM ammonium formate in MeOH (MS grade, mobile phase B): mobile phase B was increased from 68 to 83% within 13 min followed by an immediate decrease to 68% mobile phase B and an abidance at this condition until 17 min. The retention time of the derivatized BMAA was determined as 8.2 min. For the subsequent MS detection the MRM mode (positive mode) will be used with m/z of 333 (Q1, derivatized BMAA) measuring the transition to product ions m/z 273 and 245. Calibration was linear (R2 = 0.999) between 1 and 100 μg/L. Limit of detection (LOD) was 1 ng/mL derivatized BMAA. Quantification of derivatized BMAA was conducted with the internal standard homo-arginine (included in the kit) allowing the consideration of derivatization efficiency [54,10].
Chromatographic separation of CYN was achieved with the Kinetex HILIC column (2.6 µm, 2.1 x 100 mm) on the same equipment as for the MC-LR. The column oven temperature was set to 35° C and the injection volume used was 20 µL at a flow rate of 0.5 mL/min. A gradient elusion was used starting at 95% acetonitrile (MS grade) for 5 minutes which was then decreased to 50% over 3 minutes with a post time of 2 minutes, resulting in a retention time of 4.2 min for CYN. For the subsequent MS– MS detection the MRM mode (positive mode) will be used with a mass transfer of 416 (Q1) and 176 and 194 (Q3) for CYN. Calibrations for this method were linear (R2 = 0.998) between 0.01 and 100 μg/L with a LOD of 2 pg on column.
The effect of MC-LR, BMAA and CYN on the growth and biosorption potential of M. hiemalis EH5 was analyzed using the SPSS Statistics software (α= 0.05, 95% CI). A Shapiro-Wilk’s test (p>0.05) showed that the values (radial growth extension, dry weight and uptake) were with some exceptions (explained below) normally distributed, and ANOVA tables of different responses (radial growth extension, dry weight and uptake) were used to evaluate the factors (p>0.05). Data, which did not follow a normal distribution (growth at day seven and biomass determined via dry weight of CYN 250 µg/L), was analyzed with non-parametric tests, such as the Kruskal-Wallis and Mann-Whitney-U-test (p>0.05). Uptake was tested with the t-test (p<0.05).
The removal of cyanobacterial toxins from water bodies is fundamental to maintain human and ecosystem health. This research focuses on three cyanotoxins, which occur worldwide and differ in their mode of action: the hepatotoxin MC-LR, the neurotoxin BMAA and the cytotoxin CYN.
As a first step, inhibition zone assays were conducted to study the sensitivity of M. hiemalis EH5 towards MC-LR, BMAA and CYN. Data of three independent replicates and photos taken after three days of incubationare shown in Table 1 and Figure 2 (A-C show 100 μg/L MC-LR, BMAA and CYN respectively). M. hiemalis EH5 appears to be resistant to all of the cyanotoxins tested, which is underlined by the lack of inhibition zone formation (Figure 2A-C) compared to the positive control antimitotic (±)-miconazole nitrate salt (Figure 2D). In A-C the fungus shows fast adaptation and clearly grows over the toxic impregnated filter zones without expressing any sensitivity towards MC-LR, BMAA or CYN. D shows an example of the positive control, where a clear zone formation of 14.3 ± 0.6 mm was observed.
This behavior gives evidence that fungal growth is not affected and that M. hiemalis EH5 is able to grow in the presence of the tested cyanobacterial toxins.
Growth characteristics were examined in more detail in petri dishes, where the agar surface was coated homogenously and separately with the pure MC-LR, BMAA and CYN toxin solution in order to elucidate the effect on the growth rate constants of M. hiemalisEH5 in the presence of MC-LR, BMAA and CYN at various ecologically relevant concentrations [56]. The maximum concentration was chosen according to the highest cyanotoxin concentration reported in the blooms in Lake Chaohu, China [57] and the lower concentration related to the lower doses occurring in German fresh waters (up to 119 μg/L of MC-LR in Berlin water bodies, [56].
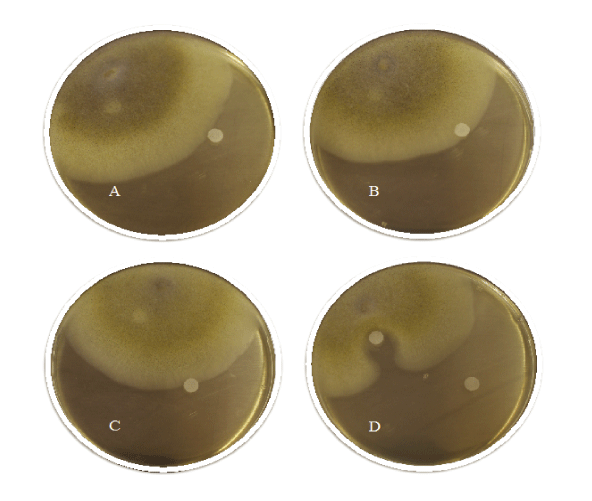
Figure 2: Inhibition zone assay as an adaptation of the Kirby-Bauer disk diffusion test of the cyanobacterial toxins MC‑LR (A), BMAA (B) and CYN (C) compared to the control (±)‑miconazole nitrate salt (D).
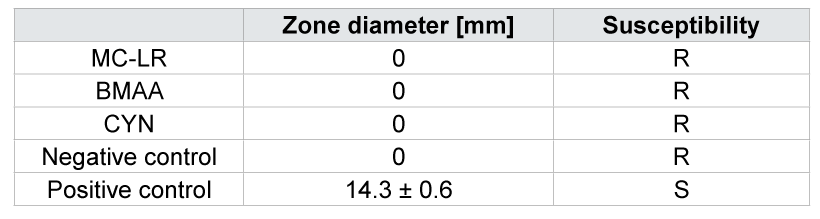
Table 1: Zone diameter of the inhibition zone assays and characterization of susceptibility. Data are means ± SE. Water was used as a negative control and (±)‑miconazole nitrate salt as positive control (R = resistant, S = susceptible).
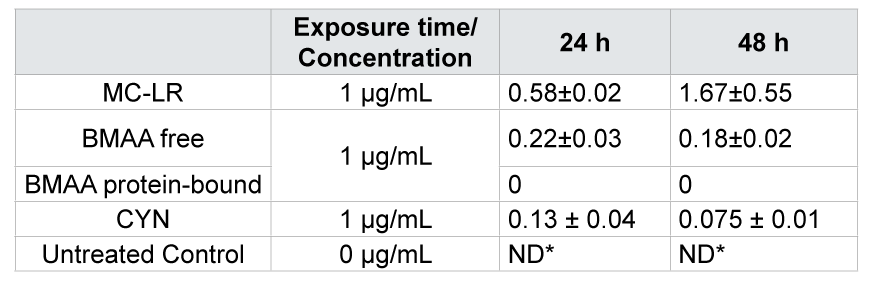
Table 2: Mycelial concentration (mg per g dw ± SE) of MC-LR, BMAA (free and protein-bound) and CYN after exposure of M. hiemalis EH5 to 1000 µg/L for 24 and 48 hours. Values are means ± SE. Significant differences (ANOVA, t-test, p < 0.05) between treatment and control of both sampling dates with no timedependency (p > 0.05) were observed. *ND ‑ not detected.
The radial extension of the culture was monitored daily and plotted versus time. White-gray colonies of M. hiemalis EH5 were expanding circularly at constant rates of 11.2 ± 0.5 mm per day. The linear growth profiles are shown in Figure 3A-C.
Data, expressed as the distance in mm, were analyzed at all time points separately for each concentration of the individual toxin compared to the control (without addition of cyanobacterial toxins). Within the whole concentration range (5 µg/L - 1000 μg/L), no significant difference was observed (ANOVA, p>0.05) at each time point, showing that the growth of M. hiemalis EH5 was not negatively affected by exposure of the fungus to increasing concentrations of MC-LR, BMAA and CYN. Even at maximum exposure concentration, the fungus continued to grow and no toxic impact was observed. Where maximum extension was reached before the end of the experiment, the distance value from the inoculum to the plate wall (80 mm) was taken for calculations and graphical illustration; the bars are marked with stars in Figure 3A-C.
To reveal more precise information about the effect of cyanobacterial toxins on mycelial development and productivity, aerial growth was considered as well. Additionally to lateral growth, the biomass production was determined via dry gravimetric analysis.
After seven days, when the fungal colonies had reached diameters of between 70 and 80 mm, dry weights were recorded. Statistical Shapiro tests showed normal distribution of the dry weight values, with the exception of CYN at 250 μg/L. The obtained data set was analyzed with ANOVA, and the evaluated toxin and concentration dependent responses did not show any significant deviations compared to the control (p>0.05). For CYN at 250 μg/L, the non-paramagnetic Mann-Whitney-U-test evidenced the same hypothesis (p>0.05) when comparing the data set to the control.
The cyanobacterial toxins neither showed to affect biomass production of M. hiemalis EH5 nor to negatively influence mycelial growth after a seven days exposure (Figure 4).
Concentration-depending dry weight results were tested for all the toxins independently proving that there is no significant deviation from the control within the whole concentration range (ANOVA, p > 0.05 and Kruskal-Wallis/Mann-Whitney-U-test, p>0.05).
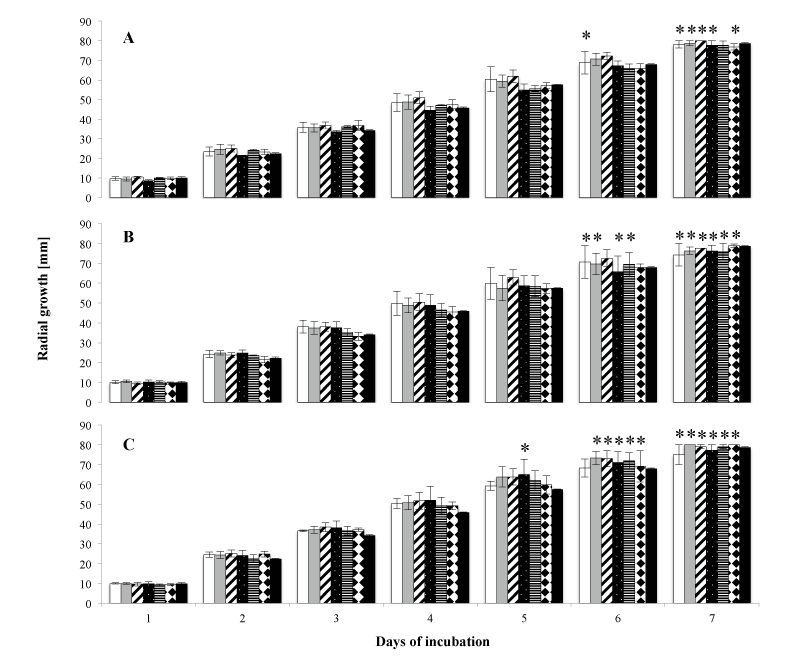
Figure 3: Concentration-dependent growth kinetic of M. hiemalis EH5 in the presence of the cyanobacterial toxins MC‑LR (A), BMAA (B) and CYN (C) at different concentrations (bars are white 5 μg/L, grey 50 μg/L, diagonally striped 100 μg/L, dotted 250 μg/L, horizontally striped 500 μg/L, squared 1000 μg/L and black control). Data are means ± SE of three independent replicates. * indicate maximal periphery, where the maximum distance of 80 mm was reached, and this value was used for calculations and graphical illustration. No statistical differences were observed when comparing concentration/ toxin to the control at each time point (p>0.05).
These results indicate high tolerance to MC-LR, BMAA and CYN of the aquatic fungus, possibly depending on the capacity to degrade the toxins by specific enzymes.
Uptake experiments were conducted to examine biosorption capacity and possible fungal-toxin interactions.The extraction methods, which were applied to isolate the cyanobacterial toxins from the fungal cells, yielded adequate recoveries, with recoveries for MC-LR being above 60% and CYN above 85%. BMAA extraction and derivatization was adopted from Esterhuizen and [14] using the EZfaastTM amino acid analysis kitf or LC/MS (Phenomenex).
An appropriate amount of mycelial biomass was obtained upon growing cultures of M. hiemalis EH5 for ten days under optimal growth conditions. M. hiemalis EH5 controls not exposed to MC-LR, BMAA and CYN contained no cyanobacterial toxin after a period of 24 and 48 h (Figure 5). The highest used concentration from the growth experiment (1000 μg/L) was applied as the exposure dosage with an exposure time of 24 and 48 h in five replicates for each toxin separately. All the three cyanobacterial toxins tested were taken up by M. hiemalis EH5 (Figure 5) showing good biosorption capacity. After 24 h exposure time, toxin levels were observed in the milligram per gram range and were still detected after 48 h in the mycelium of M. hiemalis EH5. However, no time-dependent uptake was observed, and therefore no statement on the rate of uptake/efflux and possible bioaccumulation can be made. After 24 h exposure, a maximum amount of 0.58 mg MC-LR per gram mycelial biomass (dw) was taken up in the vegetative part of M. hiemalis EH5, the BMAA and CYN level detected was 0.22 and 0.13 mg toxin/g dw respectively. After a prolonged exposure of 48 h, no significant alterations in the biosorption pattern were observed, when the data for each single toxin was compared at the different exposure times (p>0.05). However, mycelial biomass exposed to MC-LR was found to contain significantly greater toxin concentrations than mycelia exposed to BMAA and CYN respectively (p<0.05), whereas between the BMAA and CYN uptake extent, no significant difference was observed (p>0.05). The uptake requires that the toxin penetrates the fungal cell and its efficiency is attributed to the specific structure of the cell wall with chitin and chitosan as main constituents in the cell walls of fungal organisms. Depending on their chemical properties, molecules may be absorbed by either passive diffusion or active transport. The hydrophobicity of MC-LR may in part be a possible explanation for the enhanced toxin uptake in M. hiemalis EH5 mycelial cells, as the chitin cell wall is highly hydrophobic, and hydrophobic molecules may therefore have a facilitated entrance into the cells as they may interact more easily with the lipophilic cell wall. BMAA is highly hydrophilic, but as a small molecule consisting of only one amino acid, it may be taken up by simple diffusion or alternatively be transported into the cell by one of the amino acid carriers; the similarity of BMAA to glutamate connected with its agonistic activity on the glutamate receptor has been reported and may explain a possible uptake route for the toxin in fungal mycelia. As BMAA exists in plasma in several forms (neutral, zwitterion, tripolar cation, and α- and β-carbamate), it is possible that each of the species may express affinity for one or more carriers. In contrast to (aquatic) plant and animal uptake mechanisms [16], no mechanism of association of BMAA with proteins was observed in the aquatic fungus, suggesting a different mode of uptake possibly accompanied by a lower toxicity on the fungal organism, as BMAA will not accumulate and be incorporated into cellular proteins. BMAA protein incorporation in mycelia might be prevented by activated defense mechanisms of M. hiemalis EH5, however, this needs to be further investigated, because the assumption of an insufficient exposure time should not be rejected either. It is possible that longer turnaround time may be needed. Despite the hydrophilic nature of CYN, making the molecule unlikely to cross cell walls, its small molecular weight makes passive diffusion nevertheless a considerable probability [58].
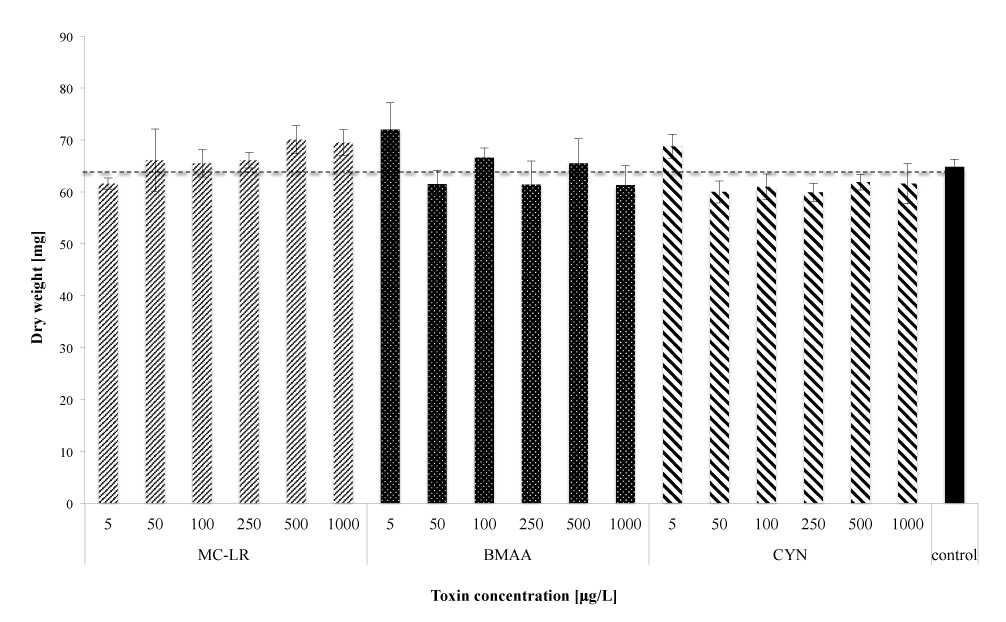
Figure 4: Biomass production expressed as dry weights after one week of exposure. Data are means ± SE of three independent replicates. No statistical differences were observed when comparing concentration/toxin to the control (p>0.05).
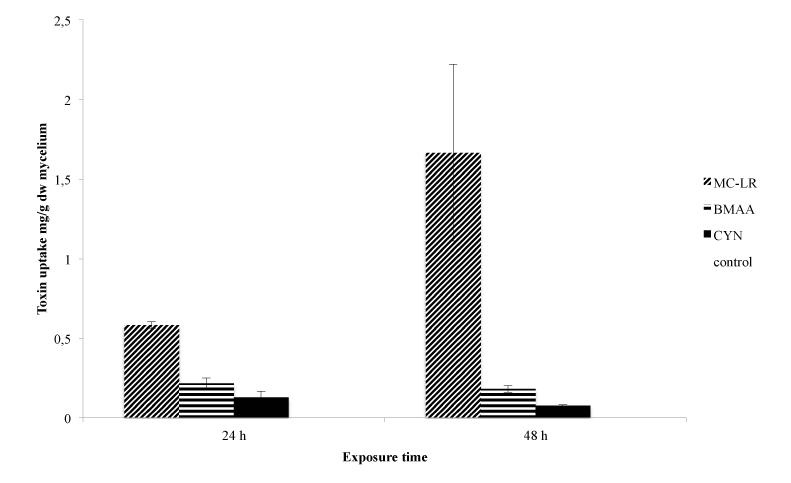
Figure 5: Total toxin uptake by M. hiemalis EH5 expressed as milligram toxin per gram dry weight of lyophilized mycelial biomass after 24 and 48 h exposure to 1000 μg/L MC-LR, BMAA and CYN. Data are means ± SE (n=5).
Because of the resistance of the fungal sporangiospores to the mechanical disruption methods used in this experiment, the amounts of the cyanotoxins quantified by LC-MS/MS are only attributed to the uptake of MC-LR, BMAA and CYN in the vegetative mycelial part of the aquatic fungi (Table 2). This is the first report of an uptake study of cyanobacterial toxins by aquatic fungi. Its results are very promising as the biosorption capability of M. hiemalis EH5 lies in the range and in some cases exceeds the uptake potential of several aquatic plants, which have been previously reported to effectively accumulate significant amounts of cyanobacterial toxins. An uptake of between 0.6% and 1.75% of the applied radio labeled MC-LR has been shown in the three rooted aquatic plants C. demersum, Elodea canadensis, and Vesicularia dubyana [59]. In comparison, a higher uptake percentage was observed in M. hiemalis EH5, whichshowed an uptake of between 1.95 to 2.9% of the applied MC-LR. Another study demonstrated MC-LR uptake in Lemna minor (L. minor) and Chladophora fracta (C. fracta) [60]. L. minor took up 0.288 ± 0.009 mg/gww after a 5 d exposure to MC-LR but with a 20 fold higher treatment dosage used. C. fracta reached a maximum uptake of 0.042 ± 0.015 mg/gww but when a six fold treatment dosage was applied. When comparison is restricted to the results obtained with the lower concentration, which was still three fold higher than the concentration applied to M. hiemalis EH5 in the present study, MC-LR uptake levels detected were 0.046 ± 0.007 mg/gww in L. minor and 0.041 ± 0.016 mg/g ww in C. fracta, which under these relative comparable concentration conditions are both substantially lower than the MC-LR content taken up by M. hiemalis EH5. The submerged macrophyte Vallisneria natans took up a maximum of 0.013.63 ± 0.00342 mg/gdw when exposed for 14 dto 25 μg/L MC-LR.
BMAA uptake by the aquatic fungus M. hiemalis EH5 was distinctly higher than by the model aquatic plant C. demersum. Interestingly, as stated above no protein-associated form was detected in M. hiemalis EH5, thereby showing a difference between fungal and (aquatic) plant or animal biosorption characteristics of BMAA. Both free and protein-bound BMAA was found in aquatic plants, e.g. C. demersum [54], Fontinalis antipyretica, Riccia fluitans, and Lomariopsis lineate [10], in wheat Triticum aestivum [61] and in animals, e.g. freshwater mussels [62] and rats [63].
Cyanobacterial toxins are effectively taken up by M. hiemalis EH5 and are so no longer available to food chain. More particularly, the toxins could be broken down in situ or ex situ and by this means eliminated completely by M. hiemalis EH5, and lasting removed from contaminated waters.
In summary, this study showed that the three tested cyanobacterial toxins have no toxic impact on the aquatic fungus M. hiemalis EH5 and that the organism can easily grow in their presence. No decrease in growth and biomass production was observed in M. hiemalis EH5 cultures exposed to MC-LR, BMAA and CYN for up to seven days at concentrations ranging from 5-1000 μg/L. Moreover, rapid and significant MC-LR, BMAA and CYN uptake by M. hiemalis EH5 was demonstrated. These results constitute the first report of uptake of cyanobacterial toxins by an aquatic fungus. The ability to adapt to the toxic perturbation upon
biosorption indicates a strong resistance of the water fungus M. hiemalis EH5, which is a prerequisite for its use as a bioremediation organism. Previous studies have shown the ability of M. hiemalis EH5 to breakdown the herbicide isoproturon. Possible sites to be attacked were C-C and C-N bonds [47], which are also present in MC-LR, BMAA and CYN (structures are shown in Figure 1). Therefore, it is possible, that M. hiemalis EH5 might be able to breakdown the structures of the cyanobacterial toxins as well and use the metabolites as a carbon source, which makes M. hiemalis EH5 an ideal organism to be tested for mycoremediation purposes. The utilization of the water fungus, which acts based on simple ecosystem functions in its natural habitat, might be a breakthrough in the field of biodegradation and removal of cyanobacterial toxins from contaminated water bodies and could offer an environmentally friendly and sustainable elimination process. The aquatic fungus has shown to be resistant to high toxin concentrations; this together with its known effectiveness even under extreme environmental conditions and low temperatures [47] enables a universal and season independent application. Future studies are suggested to examine metabolism and degradation ability of M. hiemalis EH5 towards cyanobacterial toxins.
We thank the Elsa-Neumann-Stipendium des Landes Berlin for financial support of Evelyn Balsano. We acknowledge Dr. V. ContardoJara for scientific input and S. Kühn for technical assistance in the research.
Download Provisional PDF Here
Article Type: Research Article
Citation: Balsano E, Esterhuizen-Londt M, Hoque E, Pflugmacher S (2015) Toxin Resistance in Aquatic Fungi Poses Environmentally Friendly Remediation Possibilities: A Study on the Growth Responses and Biosorption Potential of Mucor hiemalis EH5 against Cyanobacterial Toxins. Int J Water and Wastewater Treatment 1 (1): doi http://dx.doi.org/10.16966/2381- 5299.101
Copyright: © 2015 Balsano E, et al. This is an openaccess article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Publication history:
All Sci Forschen Journals are Open Access