Opinion
The notion that the immune system is critical for lifelong control of
malignant transformation has been around for decades, and is under
scored by anecdotal observations of spontaneously regressing tumors
as well as evidence that cancer incidence increases with age alongside
a waning immune system. Over the past half century research has
provided solid mechanistic evidence in support for the critical role of
the immune system in preventing cancer and has ushered in the current
era of cancer immunotherapy. The employment of the immune system to
treat or prevent cancer is commonly referred to as immunotherapy and is
comprised of two overarching categories, passive and active [1]. Passive
immunotherapy largely involves the administration of specific antibodies,
cytokines or T cells. Indeed, passive administration of specific-T cells or
of monoclonal antibodies against the T cell inhibitory receptor CTLA-
4 and more recently against the death receptor PD-1/PD-L-1, which is
collectively referred to as immune checkpoint blockade, has recently
gained international acclaim [2], though not without immune related
adverse effects [3]. Active immunotherapy can be most easily defined by
vaccination. While passive immunotherapies often engage the immune
system independent of the knowledge of defined tumor antigens (an
exception being some forms of adoptive cell therapy [4], vaccines elicit
antigen-specific immune responses by targeting tumor associated antigens
[5]. This is not to say that the immune responses elicited by checkpoint
blockade are anything but specific. It is becoming clear that tumor
associated antigen-specific T cells are elicited following administration
of antibodies to immune checkpoints underscoring the widely accepted
belief that specificity is critical for immune therapy of cancer. It should be
emphasized here that passive forms of immunotherapy have rightly taken
center stage at this time, and have proven as effective as current standard
forms of treatment if not more effective, particularly for malignant
melanoma. However passive approaches are by no means a cure. In this
light, two overarching questions must be addressed. Why have vaccines
against cancers lagged behind the advancement of passive therapies in
clinical trials? And, will vaccines that target defined tumor-associated
antigens emerge as the next generation immunotherapy?
To address these questions it is necessary to first identify the best choice
for tumor antigens to target and second to define some surmountable
obstacles impacting the success of vaccines. Given the indigenous power
with which the immune system is regulated against self-recognition and
response, early candidates for vaccine antigen targets were logically those
farthest from self-proteins and normal self-expression. Viral antigens,
mutated antigens, and tissue-restricted antigens were demonstrably the
most immunogenic and most foreign relative to the host. For those not
so common cancers with viral etiologies, cervical carcinoma being the
exception in regard to incidence and mortality, vaccines are proving
to be powerful. However, for the most common and most lethal solid
malignancies, lung cancer, breast cancer, colon cancer and prostate
cancer for example, there appears to be no virus association and few to
no mutated proteins that are critical for the cancer to survive. This may
have been perceived as a dilemma a decade ago and indeed impaired the
progress of cancer vaccine development, only recently has targeting selfproteins
with vaccines against cancers been realized. Proteins that are
self, non-mutated with wide tissue expression, but overexpressed or up
regulated in malignant cells compared to the normal counter parts include
telomerase, survive in and most recently Tumor Protein D52 (TPD52)
[6]. Importantly, TPD52 is involved in initiating and maintaining the
malignant state [7] and thus critical for the cancer cells to survive [8].
Tumor associated antigens such as these are being classified as over
expressed oncogenic tumor-self antigens and may represent the spearhead
of the next generation of vaccines against cancer [9]. Preclinical studies
have demonstrated that vaccine induced immunity against TPD52 is
effective against prostate cancer and sarcomas in murine models, without
inducing autoimmunity against healthy tissues and cells [10-13]. A
powerful and important characteristic of these antigens is their universal
or near universal over expression in a large number of cancers making
the clinical administration of vaccines against them wide spread in
application [6,9].
It is arguable that the early years of cancer vaccine development,
though driven by sound rationale, were largely an effort to ascribe to a
cancer an antigen that would be immunogenic, i.e. looking for viruses in
multiple cancers. In hindsight this was likely an early obstacle to vaccine
development given the time and effort spent without success for most solid
malignancies. In contrast, recent efforts have focused on asking cancer
cells to reveal their content of candidate antigens whether self in nature
or not. This approach required investigation in spite of the dogma that
tolerance would not allow a vaccine to elicit an immune response against
a self-protein even if over expressed, a second obstacle that had to be
overcome. The development of high throughput genomic and proteomic
technologies clearly facilitated the new recent era of tumor antigen
discovery through differential gene expression analyses. A third obstacle
was the concerted effort and time spent developing more potent vaccine
vehicles to make targeting of poor antigens more immunogenic (again
poor antigen is no longer ascribed to self- proteins that are overexpressed
and indispensable to the tumor). Again this effort was undergirded by
sound logic and has delivered some very powerful formulations that
will be useful in the near future. However, this overall effort was another
setback to vaccine development due largely to the use of poor antigens
in the innovative and potent vehicles, this supports the notions that in
the end it’s the antigen(s) that are the most important component of the
vaccine and the cancer cells decide what those antigens are. Notably,
vaccines comprised of protein with chemical and/or molecular adjuvants,
antigen pulsed dendritic cells, or plasmid DNA delivered by common
established routes proved to be effective when the right antigen was
included [9]. Finally, and likely the most difficult obstacle to overcome
is clinical timing of vaccine administration. Most if not all clinical trials
involving cancer vaccines are approved for the late stage therapeutic
setting, this is understandable relative to patient safety. However, even
vaccine trials against completely foreign pathogenic microbes would
be just as disappointing as many cancer vaccine trials have been to
date if they were administered therapeutically in the presence of full on
infectious disease. Small pox may still be a serious health issue. Perhaps
with rapid advances in early cancer detection technologies, refinement
of genetic monitoring and diagnostics, and the reality of personalized
medicine, vaccine trials will be approved for low to no tumor burden
cases with demonstrable risk. With continued study and development of
the newest overexpressed oncogenic tumor-self antigens as vaccine targets
administered perhaps in combination with passive cell transfer therapies
or immune checkpoint blockade, it will be realized that vaccination is the
next generation immunotherapy for solid malignancies (Figure 1).
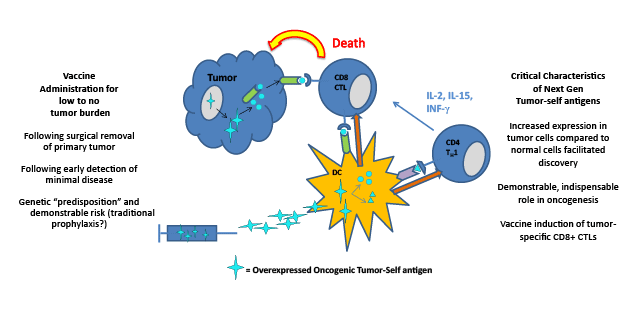
Figure 1: Cancer Vaccination Targeting: Over expressed Oncogenic Tumor-Self Antigens
The changes defining next generation cancer vaccines that will result in greater clinical success include the character of the antigenic target and the
timing of administration. It is arguable that the early years of cancer vaccine development were largely an effort to ascribe to a cancer an antigen that
might be immunogenic and foreign relative to the host. More recent efforts have focused on asking cancer cells to reveal their content of candidate
antigens whether self in nature or not. Overexpressed oncogenic tumor-self antigens may represent the spearhead of the next generation of cancer
vaccines. Rapid advances in early cancer detection technologies, refinement of genetic monitoring and diagnostics and the reality of personalized
medicine, will usher in vaccine trials approved for low to no tumor burden cases with demonstrable risk of developing clinically relevant disease, a
scenario that will yield astounding progress. Traditional prophylactic vaccination for most cancers may not be attainable and may not be necessary.
Download Provisional PDF Here
Article Information
Article Type: Opinion Article
Citation: Bright RK (2015) Cancer Vaccines: The Next
Generation Immunotherapy. Int J Vaccine Immunizat
2(1): doi http://dx.doi.org/10.16966/2470-9948.105
Copyright: © 2015 Bright RK. This is an openaccess
article distributed under the terms of
the Creative Commons Attribution License,
which permits unrestricted use, distribution, and
reproduction in any medium, provided the original
author and source are credited.
Publication history:
Received date: 04 Dec 2015
Accepted date: 23
Dec 2015
Published date: 30 December 2015