
Figure 1: Schematic representation of identifying drug target against microbes.


Umadevi S1 Ayyasamy PM2 Rajakumar S1*
1Department of Marine Biotechnology, Bharathidasan University, Tiruchirappalli, India*Corresponding author: Rajakumar S, Department of Marine Biotechnology, Bharathidasan University, Tiruchirappalli, India, Tel: +91-431- 2407113; E-mail: kodairaj@gmail.com
Granular conjunctivitis is one of the leading causes of infectious blindness worldwide. It is caused by Chlamydia trachomatis bacterium which produces a characteristic roughening in the inner surface of the eyelids. Though antibacterial drugs have been identified so far, none give the successful remedy. In recent years, genome-sequencing projects of pathogens and bioinformatic techniques have revolutionized microbial drug target identification. In this work, codon adaptation index (CAI) was used as a measure to predict the frequency of codon usage in the highly expressed genes and was coupled with other protein sequence analysis for mining potential drug targets. The chosen genes were filtered against non-homologous to human proteins. The functional significance, sub-cellular location and other parameters were used to narrow down the target. On the other hand, the drug molecules were screened from marine secondary metabolites. The compounds were collected from literature and were screened for drug-likeness property. Five therapeutic targets were identified from the results, among ATPase DnaA and DNA polymerase III subunit alpha could be the good drug targets as they are involved in essential functionalities like DNA replication and regulation. Also, for quick permeable drugs, oligopeptide-binding proteins namely replicative DNA helicase, DNA polymerase-I and protein translocate subunit Sec A were identified. The molecular docking studies of those marine compounds with the therapeutic target reveals that chloriolin A from Jaspis marine sponge may act effective against C. trachomatis.
Granular conjunctivitis; Ocular pathogen; Chlamydia trachomatis; Therapeutic target; Marine metabolites; Bioinformatics
Granular conjunctivitis commonly known as trachoma is caused by the ocular pathogenic bacterium Chlamydia trachomatis, which spreads through contact with eye discharge from the infected person and transmission by eye-seeking flies [1]. Globally 8 million people have permanent blindness due to trachoma; further 1.8 million have low vision. About 40 million people have trichiasis, if untreated this condition leads to the formation of irreversible corneal impairments, produces a characteristic roughening of the inner surface in eyelids and blindness [2,3]. In similar environment, among individuals variability in trachoma progression reveals that host genetic factors influence outcome. The host factors including human leukocyte antigen (HLA) haplotypes, gene polymorphisms in interferon (IFN)-γ, interleukin-10, tumor necrosis factor α and matrix metalloproteinase 9 are mainly associated with this. Trachoma is caused by serotypes A, B, Ba, and C. The major outer membrane protein (MOMP) of C. trachomatis encoded by ompA, is the immunodominant surface antigen and this differentiates all C. trachomatis isolates into serogroups and serotypes [4].
The understanding of chlamydial pathogenesis at the molecular level has been hindered in recent years. The gene Pgp4 is a transcriptional regulator of plasmid-encoded Pgp3 and multiple chromosomal genes, including the glycogen synthase gene glgA, that are likely important in chlamydial virulence [5]. To inactivate whole chlamydial organism-based vaccines failed to induce protection in humans. Wang et al. [6], made an attempt to find out the expressed immunodominant Ags in humans. On the other hand, detailed phylogeny based on whole-genome sequencing of representative strains of C. trachomatis from both trachoma and lymphogranuloma venereum (LGV) was done by Harris et al. [7].
The antibiotics tetracycline and azhithromycin are widely used against Chlamydia. Mass distribution of tetracycline eye ointment in number of countries in 1950s and 1960s was also ultimately unsuccessful [8]. The single-dose azithromycin is at least as effective as a prolonged course of tetracycline [9]. It is effective in active infection only but not in eliminating active trachoma [10,11]. Recent report also reveals that, the efficacy of single-dose azithromycin may be considerably lower than a week of doxycycline for treating rectal chlamydia. However, the available evidence is very poor, randomized controlled trials are urgently required [12]. The completion of human genome project and phenomenal growth of microbial sequence databases have increased the chances of identifying potent drug targets against human pathogens. One of the important strategies to identify the novel drug target is finding the bacterial genes that are non-homologous to human genes and important for the survival of bacteria. A subtractive genomics approach and bioinformatics provide opportunities for finding the drug targets against pathogens [13]. Subtractive genomics was successfully utilized by several authors to find the novel drug targets in Pseudomonas aeruginosa [14,15], Helicobacter pylori [16], Mycobacterium tuberculosis [17], Neisseria species [18,19]. Besides, the drug targets were identified based on the phylogenetic analysis [20] and on mouse model phenotype [21].
In recent years, many bioactive compounds have been extracted from various marine animals like tunicates, sponges, soft corals, sea hares, nudibranchs, bryozoans, sea slugs and marine organisms [22,23]. Also the development of new diving techniques, remote operated machines, etc. makes the collection of marine samples easily from depths of the sea [24]. In this study an attempt has been made to identify the therapeutic targets in C. trachomatis by means of in silico analysis. Also this study focused to find out the marine secondary metabolite that could inhibit the identified therapeutic target in C. trachomatis.
Collection of gene sequences: The list of protein coding genes of C. trachomatis were filtered in the NCBI (National Center for Biotechnology Information) database. Then T-iDT (http://www.milser.co.in/research.htm) was used to filter the essential genes, which are required for the survival of the organism. For those genes, sequences were retrieved from NCBI in FASTA.
Selection of significant genes: The significant genes were selected on the basis of the frequency of codon usage in the highly expressed genes using codon adaptation index (CAI). This index uses relative fitness values for each synonymous codon; expressed as the geometric mean of relative adaptiveness values of each codon and calculated by using the equation, CAI=exp(1/L∑log(wi (l))), where wi = fi /max(fj); i, j ∈synonymous codons for amino acid. This can be stated as the ratio between the observed frequency of the codon fi and the frequency of the synonymous codon fj for the particular amino acid present in the sequence. This value ranges between 0 and 1, higher the value suggests that the gene of interest likely to be highly expressed [25]. For each amino acid this value was computed by CAI Calculator 2 with 99% confidence and Morker method was used for probability calculation [26].
Selection of non-homologous human protein sequences: The corresponding protein sequences of the genes that were filtered out in the previous step were retrieved from KEGG (Kyoto Encyclopedia of Genes and Genomes) database. The proteins of C. trachomatis were compared with the host Homo sapiens protein sets to identify the unique targets for the development of effective drugs. The selection of non-homology human genes avoids the effect of drug molecules on the human proteins. For this purpose, the selected proteins were subjected to BLASTP search against Homo sapiens using threshold expectation value (E-value) of 10-3 as parameter to find out the pathogens specific to C. trachomatis [14].
Identification of PHI proteins: To identify the pathogenic proteins which have physical interaction with human proteins, the HPIDB database was used. This database integrates experimental information of protein- protein interactions (PPIs) from several public databases into a single, nonredundant web accessible resource and allows searching for homologous host-pathogen interactions. Blosum 60 matrix and the E-value cut off 1x10-6 were used for sequence similarity search with other pathogenic proteins [27].
Identification of functional significance and sub-cellular location: The potentiality of the targets was validated by obtaining functional significance using InterProScan and analyzed the metabolic pathway and sub-cellular localization by PSORTb v3.0.2 server [28]. The overall methodology was illustrated in Figure 1.
3
Figure 1: Schematic representation of identifying drug target against microbes.
Retrieval and screening of marine metabolites as drug hits: The secondary metabolites from marine organisms were collected for the purpose of screening antibacterial activity. The compounds were collected by electronic search in PubMed, Google Scholar, Web of Science using keyword (marine, ocean, drugs, pharma, medicine) search with ‘AND’, ‘OR’ Boolean operators. The references in the studies were also reviewed to check through manual searches to find other potential metabolites. The structures were got from ChemSpider, PubChem and eMolecules in ‘mol’ or ‘sdf ’ formats based on the availability. The drug-likeness was evaluated by DruLiTo (http://www.niper.gov.in/pi_dev_tools/DruLiToWeb/ DruLiTo_index.html) as it reduces the chances of selecting the false positive results. Threshold value for each parameter (drug-likeness properties) was set based on different rules to filter out the ligands. The value of molecular weight (MW) and atom molar refractivity (AMR) were preferred in the range of 230-290 and 70-110 respectively based on CMC-50 like rule. Lipinski’s rule was used to set the parameter ranges for octonal/ water partition (LogP) (≤5), hydrogen bond acceptor (HBA) (≤10) and hydrogen bond donor (HBD) (≤5).
For computed fragment based logP (AlogP) the range was taken from -0.40 to 5, the number of atoms (nAtom) was chosen as 20-70 and the number of aromatic rings (nAromRing) was taken ≤3 based on Ghose filter. Veber filter was used in setting the parameter range for total polar surface area (TPSA) (≤140). Number of rotatable bonds (nRB), ring count (RC) and number of rigid bonds (nRigidB) were chosen as ≤6, ≤3 and ≤18 respectively, according to MDDR likeness rule. BBB likeness rule helped to fit the total number of hydrogen bonds ≤8, as well the number of acetic group (nAcetic) and structural alerts (sAlerts) for carcinogenicity was set as zero [29].
Quantitative estimate of drug-likeness (QED): To quantify drug-likeness properties of compounds, value based quantitative estimate of drug-likeness (QED) was used. It results the combination of the individual desirable functions achieved by taking the geometric mean of the individual functions, as given in equation,
QED=exp(1/nΣ log(di))
where di is a series of desirability functions includes MW, AlogP, HBA, HBD, TPSA, nRB, nAromRing and sAlerts. The marine metabolites that obey all the rules and have QED>1 were selected for docking study [30].
Docking of targets with marine secondary metabolites: The 3D structure of target protein was modeled using Phyre 2 (http://www.sbg.bio.ic.ac.uk/phyre2/html/page.cgi?id=index), energy minimized and validated by Ramachadran plot using RAMPAGE at http://mordred.bioc.cam.ac.uk/~rapper/ rampage.php. Using Autodock server 4.0 simulation program, all missing hydrogens/side chain atoms in crystallography tension were added and was minimized. Gasteiger charges were calculated and non-polar hydrogen was merged to carbon atoms. The binding site was determined by DEPTH server [31]. Based on the residues (amino acids) involved in binding site, the grid box was set in AutoDockTools [32] using thumb wheel adjustment. Twelve runs were generated by using Lamarckian genetic algorithm searches. Default settings were used with an initial population of 150 randomly placed individuals, a maximum number of 2.5×107 energy evaluations and a maximum number of 2.7×104 generations. A mutation rate of 0.02 and a crossover rate of 0.8 were chosen. Results with less than 0.5 Å in positional root-mean-square deviation (RMSD) were clustered together and the results of the most favorable free energy of binding were selected. To identify the biologically active ligand (marine metabolite) with significant binding affinity and lower interaction energy were selected. These selected molecules would have the drug-likeness properties and also can inhibit the activation of selected therapeutic target.
A total of 76,250 genes of C. trachomatis A2497 strain were acquired from NCBI database. Among them 981 genes are essential for the survival of cell and genes with CAI greater than or equal to 0.75 were chosen as the significant genes. Number of genes in each CAI range and the percentage of significant and non-significant genes based on the frequency of occurrence of codon bias were represented in Figure 2. Majority of significantly expressing genes have CAI in the range of 0.75- 0.8 and 3.87% of genes were much highly expressed with the CAI value in between 0.81-0.95. In total 58.20% of genes show significance expression level.

Figure 2: CAI range of amino acids in C. trachomatis.
The protein sequences of the selected 571 genes were retrieved from KEGG database for the further proteome analysis. BLASTP has been used for this purpose with the cut off E-value 10-3. The selected list contains 465 proteins which are unique to C. trachomatis. There were 465 proteins found as non-homology to human proteins. Among them 91 were identified as redundancy and excluded. For the filtered list, the network topology of protein-protein interactions (PPIs) between the human and C. trachomatis proteins was represented in Figure 3. In the network, the degree of each human protein clearly indicates more number of proteins of pathogen interacts with a single human protein. There might be functional similarities among those pathogenic proteins (Figure 4). In this phase, 90.72% of the total essential proteins were filtered out. As more number of proteins of C. trachomatis tics were physically binding with host, it is necessary to focus on functionality of those proteins in order to identify the target for therapeutic purpose.

Figure 3: Network pattern of host-pathogen PPIs.

Figure 4: Number of genes in each parameter to narrow down the target.
Among the selected proteins, ATPase DnaA and DNA polymerase III subunit alpha could be the good drug targets as they are involved in DNA replication and regulation earlier report by Chene [33] revealed that the inhibitors for various ATPases act as a drug molecule. DNA polymerase III alpha subunit was reported as a potential drug target against M. tuberculosis [34]. However, for quick permeable drugs, Oligopeptide-binding proteins named protein translocate subunit Sec A can be a target as it is located in periplasmic membrane, also involved in ion transport. Next to these, replicative DNA helicase, DNA polymerase-I which are involved in replication and ABC transporter permease, protein translocate subunit which are involved in protein export, bacterial secretion system are fulfilling the criteria for a drug target [35]. Compared with later two, protein translocate subunit SecA was effective as it is involved in protein export, bacterial secretion system which notably involved in resistance to therapeutic drugs [36]. In addition, they are involved in ATP binding, which is the source of energy and phosphate to the cell (IPR000185). Also, the information from Drug Bank informs that the earlier identified drugs namely tetracycline, deocycyclin, erythromycin, cotrimoxazole and azithromycin which were ineffective are targeting the growth/replication of the bacteria but not targeting resistance related proteins. Based on this, in the present study protein translocate subunit SecA was chosen as target for the identification of quick permeable drug against C. trachomatis.
Thousand nine hundred fifty three metabolites were retrieved from literature study and named as MC1 – MC1953. Based on the structure the activities of the molecules were predicted. The calculated values of MW, AMR, logP, AlogP, HBA, HBD, nAtom, nAromRing, nRB, RC, nRigidB, nAcetic and sAlerts were subjected to QED values and were graphically represented in Figure 5. Even though 8 compounds have QED>1, only two metabolites were identified with satisfying all the criteria for drug-likeness. The drug leads identified were asperic acid (MC216) from Aspergillus niger and chloriolins A (MC377). The isolated from sponge Jaspis aff. Johnstoni. The drug-likeness properties of them were given in Tables 1 and 2.

Figure 5: QED of marine metabolites.
| Gene No. | Protein | Function | Cellular location |
| CTO_0216 | Oligopeptide binding protein | Transporter activity | Periplasmic membrane |
| CTO_0191 | Oligopeptide binding protein | Transporter activity | Periplasmic membrane |
| CTO_0595 | DNA polymerase III subunit alpha | DNA binding, DNA-directed DNA polymerase activity, DNA replication | Cytoplasmic membrane |
| CTO_0545 | Replicative DNA helicase | DNA binding, DNA helicase activity, ATP binding, DNA replication | Cytoplasmic membrane |
| CTO_0540 | DNA polymerase I | DNA binding, DNA-directed DNA polymerase activity, DNA replication | Cytoplasmic membrane |
| CTO_0272 | ATPase DnaA | DNA binding, DNA replication origin binding, ATP binding, DNA replication initiation, Regulation of DNA replication | Cytoplasmic membrane |
| CTO_0262 | RecR protein | DNA binding, DNA repair, DNA recombination | Cytoplasmic membrane |
| CTO_0079 | DNA replication and repair protein | Single-stranded DNA binding, ATP binding, DNA repair | Cytoplasmic membrane |
| CTO_0855 | 50S ribosomal protein | Structural constituent of ribosome, Intracellular, Translation | Cytoplasmic membrane |
| CTO_0931 | ABC transporter permease | Transporter activity | Cytoplasmic membrane |
| CTO_0490 | Protein translocase subunit | Intracellular protein transport, P-P-bond-hydrolysis- driven protein transmembrane transporter activity | Cytoplasmic membrane |
| CTO_0303 | Electron transport complex protein | Membrane transport | Cytoplasmic membrane |
| CTO_0504 | Metallo-phosphoesterase | Hydrolase activity | Golgi apparatus |
Table 1: Function and cellular component of selected proteins.
| Property | Condition | MC216 | MC377 |
| MW | 230-290 | 255.98 | 252.97 |
| LogP | ≤5 | 2.349 | 0.732 |
| AlogP | -0.40–5.6 | 0.623 | 0.759 |
| HBA | ≤10 | 4 | 3 |
| HDB | ≤5 | 0 | 0 |
| TPSA | ≤140 | 26.3 | 17.07 |
| AMR | 70-110 | 76.85 | 70.74 |
| nRB | ≤6 | 6 | 3 |
| nAtom | 20-70 | 20 | 20 |
| nAcidicGroup | 0 | 0 | 0 |
| RC | ≤3 | 1 | 2 |
| nRigidB | ≤18 | 14 | 16 |
| nAromRing | ≤3 | 0 | 0 |
| nHB | ≤8 | 4 | 3 |
| sAlerts | 0 | 0 | 0 |
Table 2: Drug-likeness properties of MC216 and MC377.
As there was no determined structures for protein translocate subunit Sec A in protein data bank, the 3D structure was predicted and illustrated in Figure 6. The stereo-chemical quality and accuracy was evaluated by Ramachandran plot (Figure 7), the result showed that 89.6% of amino acids in favored region, 10.1% in allowed region and 0.3% in outlier region. Based on the plot, the structure has been taken for the interaction study in structure based drug design module.

Figure 6: 3D structure of protein translocate subunit Sec A.

Figure 7: Ramachandran plot of modeled protein.
The active site of energy minimized modeled protein was identified; the amino acid composition of the site was represented in Figure 8. The docking results shows that, asperic acid (MC216) interacts with SecA protein by hydrogen bond with Lys22 (Length: 3.38 Å, Energy: -1.08 kcal/ mol) and with Lys108 (Length: 2.90 Å, Energy: -2.50 kcal/mol). Also the steric interaction was found with Arg19, Asp60, Glu26, Ile104, Leu23, Leu63, Lys61 and Pro64. The total binding energy is -5.37 kcal/ mol with inhibition constant 115.78 uM (Figure 9A and 9C). Chloriolin A (MC377) made hydrogen bond with SecA protein at Leu862 (Length: 3.49 Å, Energy: - 0.03 kcal/mol). Also the steric interaction was found with Cys858, Ile770, Ile857, Ile861, Leu925, Leu930, Lys836, Phe833, Phe837, Phe926 and Val922 due to overlapping electron clouds. The total binding energy was -8.34 kcal/mol with inhibition constant 776.03 nM (Figure 9B and 9D). The steric interaction was observed at more amino acids and can considered be a primary driving force of the molecular interaction between the protein and marine ligand molecules. In both the interaction, the part of the active site residues are involved, that adds significant to the results. These results accounts for the best pose obtained based on the energy and RMSD values. For the rest of the binding poses, the docking was unsuccessful as they have higher energy and inhibition constant. The conformations of the molecular interaction generates one of the pharmacopore model, thus the reliable interaction alone considered. Based on the binding energy and inhibition constant chloriolin A was found as significant against C. trachomatis. This compound is present in the marine sponge Jaspis aff. Johnstoni which is available in Indo-Pacific oceanic region [37]. So as it is easy to extract the compound and can be used as a medicinal product.

Figure 8: Amino acid composition of active site of protein translocate subunit Sec A.
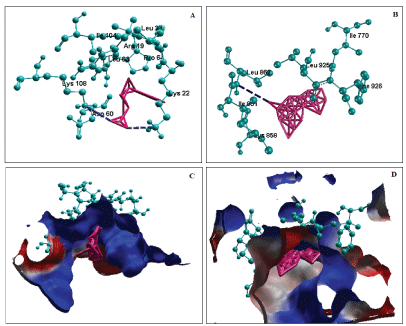
Figure 9: Docking of protein translocate subunit SecA with MC216 and MC377 (A) Hydrogen bond interaction with MC216 (B) Hydrogen bond interaction with MC377 (C) Electrostatic surface of MC216 with SecA (D) Electrostatic surface of MC377 with Sec A.
In the present study, in silico based molecular analysis of C. trachomatis traced out the essential target proteins, which are unique, highly expressing and specific to the pathogen. Protein translocate subunit Sec A was identified as therapeutic target in terms of quick permeability and also inhibition of this would suppresses the resistance against drugs. Developing drugs against this identified target, also be less or non-toxic to the host. Among the collected marine metabolites, two compounds namely asperic acid and chloriolins A satisfy all the properties of drug-likeness, further the docking result shows that the later compound is efficient than former as it has biological significant binding energy with the target, as well the inhibition constant. This study concludes that chloriolin A from Jaspis aff. Johnstoni marine sponge, which is targeting protein translocate subunit SecA can be a novel effective therapeutic compounds against C. trachomatis. Further it should be experimentally validated. Another notable plan is the combinatorial therapy of chloriolin A with already identified drugs which targets replication proteins, this may have much effectiveness.
Download Provisional PDF Here
Article Type: RESEARCH ARTICLE
Citation: Umadevi S, Ayyasamy PM, Rajakumar S (2018) In silico based Identification of Potential Therapeutic Marine Metabolites against the Ocular Pathogen Chlamydia trachomatis. J Sys Biol Res 1(1): dx.doi.org/10.16966/jsbr.102
Copyright: © 2018 Umadevi S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Publication history:
All Sci Forschen Journals are Open Access