
Figure 1: The Enterra device. This is an implantable system generating electricity, which is transmitted to the tissue via two unipolar leads. (Medtronic.com)

Rupal Patel1* Prasad Kulkarni1,2
1Division of Digestive Diseases and Nutrition, Internal Medicine Department, University of South Florida, Morsani College of Medicine, Tampa, Florida, USA*Corresponding author: Prasad Kulkarni, Division of Digestive Diseases and Nutrition, Internal Medicine Department, University of South Florida, Morsani College of Medicine, Tampa, Florida, USA, Tel: (269) 267-6151; E-mail: pkulkarn@health.usf.edu
The purpose of this review paper is to give overviews of the Enterra device, gastroparesis, the research supporting this technology, and most significantly possible means of field expansion. Some future directions that should be considered in expansion of gastroparesis management: treatment algorithms for different types of gastroparesis, endoscopic placement of Enterra, using high resolution electrical mapping of the stomach to construct a new classification scheme for human gastric electrical disorders, using EUS to obtain a fine needle aspiration biopsy of the muscular ispropria, and pyloroplasty/endoscopic pyloromyotomy in refractory gastroparesis. Also, the ultra structure of diabetic and idiopathic gastroparesis lends to the possibility of creating medical treatments to reverse or even prevent the early cellular changes specific to different types of gastroparesis. There are numerous developmental possibilities within the realm of gastric electrical stimulation, and in the treatment of gastroparesis in general.
Enterra device; Gastric Electrical Pacing
Gastric electrical stimulation (GES) has come a long way since Bilgutay et al. [1] first described it in 1963. GES is the use of electricity to stimulate nerve endings and smooth muscle within the stomach; it is used to treat refractory symptoms secondary to gastroparesis. To date, the only apparatus available and used long-term in patients is the Enterra device (Medtronic Inc, Minneapolis, MN). The purpose of this review paper is to give overviews of the Enterra device, gastroparesis, the research supporting this technology, and most significantly possible means of field expansion. The Enterra device has had a Humanitarian Device Exemption (HDE) by the Federal Drug Administration Agency (FDA) since 2000 for treatment of refractory symptoms associated with diabetic and idiopathic gastroparesis. A HDE permits for marketing of a medical device without requiring evidence of effectiveness. Devices that qualify for HDE must benefit patients with a rare disease/condition, meaning less than 4,000 patients per year. There cannot be any comparable devices already available, and the applicant must prove this is the only way to bring the device to market [2]. From 2001 to 2013, about 9,780 Enterra device neurotransmitters were sold in total, therefore on average 800 devices per year [3]. The Enterra device targets high-frequency, lowenergy stimulation to the lower part of the stomach via two unipolar intramuscular leads. Interestingly, a new model called the Enterra II device recently received FDA approval under a HDE in January 2015. Per the manufacturer, this second version has new software with an improved user interface for device programming. There will also be improvements to the system’s battery-life monitoring. Moreover, implantation has been simplified due to a customized tool [4].
In a few words, the Enterra device is an implantable system generating electricity via a neurostimulator, usually implanted in the lower abdomen below the skin, which transfers that signal to the stomach muscle via two unipolar leads (Figure 1). The initial Enterra neurostimulator was 60 mm in length, 55 mm in width, and 10 mm in depth, and the new version is 60 mm in length, 55 mm in width, and 11.4 mm in depth [4]. Currently, the neurostimulator and leads are implanted via either laparotomy or laparoscopy. Whereas lead implantation is easier with laparotomy, laparoscopy is less invasive and patients have shorter recovery periods. The two intramuscular leads are implanted into the greater curvature about 10 cm from the pylorus, and 1 cm apart from each other (Figure 2). Subsequent intra-operative gastroscopy ensures the leads have not perforated through the stomach wall. Once implanted, the device is left off for several weeks to promote wound healing. Once turned on, the neurotransmitter settings are adjusted noninvasively using a programmer (Medtronic N’Vision). Depending on the neurotransmitter settings the battery life usually lasts between 5 and 10 years. Once the battery is depleted the entire neurostimulator device must be replaced, however the same leads can be used [5] Some of the most pertinent complications associated with gastric electrical stimulator placement include infection, hemorrhage/hematoma, perforation, neurostimulator migration, lead migration, pain, and allergic reactions to implanted material [4].

Figure 1: The Enterra device. This is an implantable system generating electricity, which is transmitted to the tissue via two unipolar leads. (Medtronic.com)
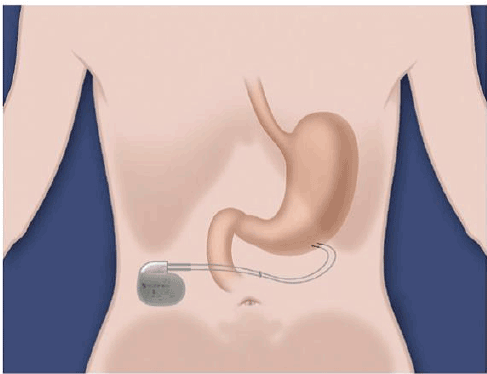
Figure 2: This illustration demonstrates placement of the leads. They are implanted into the greater curvature about 10 cm from the pylorus, 1 cm apart from each other (FDA.gov).
Gastroparesis, or delayed gastric emptying, is a debilitating condition becoming more prevalent in the United States mostly due to a growing diabetic population [6]. The three main etiologies of this disease are: diabetes (29%), post-surgical (13%), and idiopathic (36%) [7]. Gastroparesis can be difficult to treat, leading to frustration of both the patient and practitioner. The American College of Gastroenterology updated its gastroparesis management guidelines in 2013 [6]. First, once the diagnosis is confirmed with a gastric emptying study, the guidelines recommend an attempt at restoration of fluids and electrolytes, dietary modifications, and adequate glucose control. The suggested diet is a lowfat, low-residue diet consisting of soft, cooked foods, and small, frequent meals. If these initial measures are not successful, then first-line therapy consisting of prokinetic (erythromycin, metoclopramide, domperidone) and anti-emetic (anti-histamine1 receptors, 5-HT3 antagonists) pharmacotherapy is recommended. In cases refractory to these measures, the practitioner should consider feeding jejunostomy, decompressive gastrostomy, GES, or surgical therapy. Based on randomized clinical trial evidence, intrapyloric injection of botulinum toxin is no longer recommended. These new management guidelines state that GES has been shown to improve symptom severity and gastric emptying in diabetic gastroparesis, but not in idiopathic or post-surgical cases. GES should be considered for refractory symptoms, particularly nausea and vomiting (conditional recommendation based on a moderate level of evidence) [6].
Since its introduction over 15 years ago, much research has been done on the Enterra device. In 2002 Abell et al. [8] published a multicenter study in which 35 of the studied 38 drug-refractory patients had a >80% reduction in nausea and vomiting. In 2003 Abell et al. [8] published a double-blinded, randomized, crossover study of 33 patients which showed improved weekly vomiting frequency and quality of life in diabetic gastroparesis patients and all studied patients [9]. These two studies supported the FDA’s approval of Enterra’s HDE. Also, two meta-analyses have been published, one in 2009 by O’Grady et al. and the other in 2012 by Chu et al. [10,11]. The former revealed that vomiting, nausea, and total symptom severity scores all improved, decreasing from severe to mildmoderate. It included 13 studies, only 1 was a randomized controlled trial, and the remaining 12 did not have a control group [10]. The second meta-analysis also showed improvement in all three symptom severity scores. A subanalysis on diabetic, idiopathic, and post-surgical patients found the diabetic group most responsive to high-frequency GES [11]. Generally, most studies have concluded that GES improves symptoms of diabetic gastroparesis patients more than idiopathic gastroparesis patients. Further randomized, controlled studies are needed to retrieve a less biased confirmation of symptomatic improvement. Moreover, postsurgical gastroparesis patients remain to be more fully characterized in published literature.
Future Directions & Implications of Research
Like with any technology we should continue to strive for improvement, not only in the technology itself, but also in how it is utilized. As mentioned above, current GES technology appears to improve diabetic gastroparesis symptoms the most, leaving questions in the treatment of idiopathic and post-surgical gastroparesis.
On review of the literature, several expansions on the use of the Enterra device were discovered. First, defining a treatment algorithm for different types of gastroparesis would likely be beneficial. One study analyzed 22 patients who were treated with GES, but did not respond optimally to the initial neurostimulator settings. These researchers undertook an algorithmic approach to identify optimal parameters for gastroparesis of various etiologies. Interestingly, they found the postsurgical gastroparesis group required the most energy in its settings [12]. Since the pathophysiology behind different types of gastroparesis varies, the optimal neurostimulator settings likely vary as well.
Another concept is endoscopic placement of a GES device. Currently, placement of a permanent gastric electric stimulating device via endoscopy is not available. However, temporary devices are placed via endoscopy to assess whether a patient will respond to electrical stimulation prior to permanent placement via surgery [13]. One research group has designed and tested a miniature gastric neurostimulator in pigs [14,15]. Moreover, they have produced five endoscopic implantation methods of this miniature device. If we are able to translate this device to humans in the future, this would provide us with a less invasive option for placement. This device is placed into the stomach through an over tube with 2 GES electrodes attached to the gastric mucosa and secured with endoclips. This device and technique could possibly become the standard in terms of gastric stimulation for gastroparesis [14,15]. Similarly, currently the placement of stimulation electrodes for permanent gastric electrical pacing occurs via either laparotomy or laparoscopy. Endoscopic placement, however, would allow for placement without general anesthesia, which in turn would likely promote more widespread use of GES. The neutrostimulator device could be placed with local anesthesia in a subcutaneous lower abdominal pouch [16].
High resolution electrical mapping of the stomach is another techniquethat has been recently explored in more depth. This technology allows for detailed quantification and classification of gastroparesis slowwave abnormalities in spatiotemporal detail. O’Grady et al. [17] developed a wireless device which they tested on a cohort of 12 patients (6 male and 6 female, with 8 having a diabetic and 4 an idiopathic etiology). This was the first study to use high-resolution electrical mapping to examine human gastric slow-wave abnormalities in spatial detail. They did not observe a difference in the rhythm abnormalities between the diabetic and idiopathic gastroparesis groups. Aberrant slow-wave initiation was the most common class of abnormality. In 5 of 10 patients, abnormal initiation occurred at normal frequencies. Abnormalities in slow-wave conduction were observed in 7 of 12 patients, all of whom also showed abnormalities of initiation. This study emphasizes the importance of understanding the stomach’s electrical abnormality in each individual patient. Similar to high resolution esophageal manometry which better delineates esophageal dysmotility, being able to personalize stomach dysmotility testing would be groundbreaking. In the past, studies looking at rhythm disturbances have placed an emphasis on frequency abnormalities. However, this study implies that abnormal activation of patterns often occurred even at normal frequency. Highly disordered slow-wave patterns also occurred at normal frequency and with regular rhythm. Moreover, the complexity of wave propagation abnormalities in brady- and tachyarrhythmias is evidently greater than previously documented. The stomach’s body may be a common location of ectopic foci. Moreover, during initiation abnormalities, coupling of signals can lead to propagation, wave collisions, and uncoupling. Depending on where the initiation sites are located in relation to each other, these abnormalities may be/appear less or more pronounced, and variable in nature. Unstable focal events can lead to an irregular range of frequencies secondary to chaotic tissue activation and colliding wavefronts. Studying these types of variables in depth will facilitate a new classification scheme for human gastric electrical disorders [16,17].
New findings in relation to the ultra structure of diabetic and idiopathic gastroparesis lends to the possibility of creating medical treatments to reverse or even prevent the early cellular changes specific to different types of gastroparesis. In previous studies, light microscopy with immunohistochemical staining was not able to distinguish between diabetic and idiopathic gastroparesis. However, a recent study by Faussone-Pellegrini et al. [18] used electron microscopy to analyze differences in tissue from 20 diabetic gastroparesis patients, 20 idiopathic gastroparesis patients, and 20 age- and sex-matched controls. They found that there are significant characteristics distinguishing idiopathic and diabetic gastroparesis. In summary, diabetic gastroparesis is associated with a thickened basal lamina surrounding smooth muscle cells and nerves, whereas idiopathic gastroparesis biopsies displayed more intense fibrosis and more nerve damage of glial cells, nerve cell bodies, and nerve fibers. Intersitial cells of Cajal were affected in both diabetic and idiopathic gastroparesis. Understanding and studying these changes, especially changes that occur early in the disease course, will help us develop new therapies to prevent and/or minimize the early cellular changes of gastroparesis. Once we have developed targeted therapies, we could utilize individual patients’ tissue to personalize treatment according to ultrastructural findings.
Currently, biopsies are sometimes utilized to help confirm a diagnosis of gastroparesis. The standard to obtain a stomach biopsy is to obtain a full thickness sample via an invasive surgical technique. Recently, the use of endoscopic ultrasound to obtain a fine needle aspiration biopsy of the muscular ispropria has been shown to be safe, as well as yielding adequate tissue for full histologic assessment when compared to surgically obtained specimens [19]. This trial was with a small sample size (n=11), however it highlights an opportunity for a simpler and less invasive approach to stomach biopsy.
In terms of endoscopic and surgical interventions for gastroparesis, there has been some recent advancement [20,21]. Botulinum toxin injection of the pylorus was utilized relatively frequently in the early 2000s, but has since fallen out of favor. Initially, it appeared that botulinum toxin treatment did reduce symptoms and accelerate gastric emptying times. However, based on randomized control trials, current guidelines do not support the use of botulinum toxin injection of the pylorus for gastroparesis [21]. Recent studies on pyloroplasty in refractory gastroparesis, both laparoscopic and open techniques, have shown likely benefit. A recent investigation by Toro et al. [22] reported subjective improvement in symptoms of 82% patients after the procedure (n=50). Ninety percent of the study population had a nondiabetic etiology of gastroparesis. These investigators also measured gastric emptying times in these patients and found a median reduction of “time required to empty 50% of ingested meal” after laparoscopic pyloroplasty was 120 minutes with improved time in virtually all patients. Normalization (time required to empty 50% of ingested meal <60 minutes) occurred in 54% of the study population and mild delayed of gastric emptying times (time required to empty 50% of ingested meal <80 minutes) was achieved in 18% respectively [22]. Most recently, endoscopic pyloromyotomy has been investigated as a POEM-like (per-oral endoscopic myotomy) equivalent for gastroparesis. In 2013, the first case of per-oral pyloromyotomy in a patient with diabetic gastroparesis was reported [23]. In 2014, a case series of 7 patients who underwent per-oral pyloromyotomy for refractory gastroparesis reported success, with subjective improvement in symptoms in 6 of the 7 patients [24]. Endoscopic pyloromyotomy holds promise, but more studies with larger sample sizes are needed in order to solidify its place in the treatment algorithm of gastroparesis.
Analysis of gastric electrical stimulation over the last 15 years has illustrated its benefit to the treatment of refractory gastroparesis symptoms. To date, treatment has been differentiated based upon etiology of gastroparesis. Gastroparesis treatment with electrical stimulation has the possibility of becoming more personalized regardless of the underlying etiology. This will perhaps be the most successful in patients with non-diabetic etiologies of gastroparesis, as this population’s response to electrical stimulation has not been as robust as the diabetic population. In conclusion, numerous developmental possibilities in gastric electrical stimulation do exist, as well as in the treatment of gastroparesis.
None
None
Download Provisional PDF Here
Article Type: Review Article
Citation: Patel R, Kulkarni P (2016) The Enterra Device and the Future of Gastric Electrical Pacing. J Gastric Disord Ther 2(4): doi http://dx.doi. org/10.16966/2381-8689.127
Copyright: © 2016 Patel R, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Publication history:
All Sci Forschen Journals are Open Access