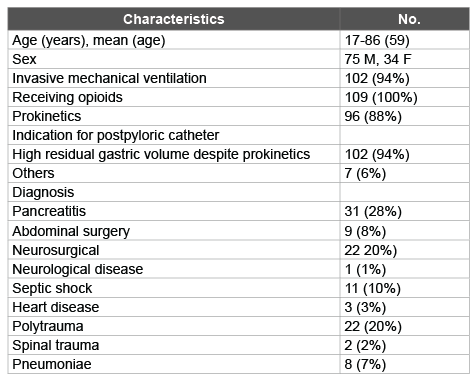
Table 1: Demographic and clinical characteristics of the 109 study patients
Marianna Zatelli1* Alessandra Carletti2 Guido dall’Acqua3 Roberta Falchi4 Mauro Cima4 Vittorio Schweiger2 Andreas Clara5
1Department of Intensive Care, Bolzano Regional Hospital, Italy*Corresponding author: Marianna Zatelli MD, Department of Intensive Care, Bolzano Regional Hospital, via Lorenz Böhler, 5, 39100 Bolzano, Italy, Tel: 390471908522; Fax: 390471908997; E-mail: marianna.zatelli@asbz.it
Background: Enteral nutrition in critical patients constitutes one of the primary aims of therapy. A number of critical patients as a result of the acute pathology in progress and of concomitant diseases, encounter difficulty tolerating enteral nutrition. The adoption of the post-pyloric route is desirable in these categories of critical patients.
Method: A multicentre observational study was conducted to evaluate the success rate and duration of the technique in different categories of critical patients. The Tiger 2 self-advancing jejunal feeding tube was used in 109 patients, ventilated and not-ventilated, requiring enteral nutrition with a diagnosis of acute pancreatitis, abdominal compartment syndrome (postsurgical or traumatic) and severe gastric stasis (≥ 500 ml) and, in the case of persistent gastric stasis, despite the administration of prokinetic drugs (erythromycin or metoclopramide) after 3 days.
Results: In 89 patients, placement of the self-advancing jejunal feeding tube was achieved at the first attempt (82%). In 7 cases (6%) it was placed at the second attempt. The median time for placement of the Tiger 2 self-advancing jejunal feeding tube according to protocol was 4 hours.
Conclusions: This multicentre observational prospective study has shown that placement of a Tiger 2 self-advancing jejunal feeding tube appears to be effective and relatively rapid in critical patients suffering from pancreatic and abdominal disease and in patients with persistent gastric stasis despite the administration of prokinetic drugs. The adoption of a placement technique considering the anatomy of the gastrointestinal tract and the pharmacological properties of prokinetic drugs seems to be fundamental.
Clinical relevance statement: The use of the Tiger 2 self-advancing jejunal feeding tube is feasible in intensive care by a team of medical and trained nursing staff complying with a specific nutritional protocol. Patients who have neurosurgical disorders can get the most benefit from this approach.
Enteral nutrition; Gastric stasis; Tiger 2 self-advancing jejunal feeding tube
Enteral nutrition constitutes an integral part of the therapy of critical patients. All critical patients need adequate enteral therapy in relation to their calorie/protein requirements, calculated on the basis of their characteristics and the acute disease in progress.
In clinical practice, critical patients suffering from a number of abdominal diseases such as acute pancreatitis and abdominal compartment syndrome receive enteral nutrition mainly via the jejunal route, as is well documented and extensively discussed in the literature[1].
There are, however, other groups of patients who would benefit from such an approach, in view of the fact that half of the critical patient’s present severe gastric stasis and unsatisfactory gastric tolerance of enteral nutrition [2].
Post pyloric nutrition is, moreover, indicated in patients at high risk of aspiration and in cases of gastric ileus in which, predictably, nutrition via the gastric route is not tolerated [3].
Four randomized clinical trials in the last 5 years have attempted to end the debate on the benefits of post pyloric feeding compared to intra gastric feeding. 2 trials demonstrated an increase in calorie and protein intake and lower incidence of PAP in patient fed via the post pyloric route [4-7].
The Canadian Critical Care Guidelines Committee had the strongest recommendation for small bowel feeding stating that, if, feasible, all critically ill patient should be fed via this route, based on the reduction in pneumonia [8,9].
When the decision is made to use post pyloric tube placement, the next step is how to safely place the device without risks for the patients and delays in starting the nutrition therapy.
The identification of an easy jejunal approach permitting the early initiation of enteral nutrition constitutes the fundamental aim of this research.
The gold standard for the placement of a jejunal feeding tube is the endoscopic guided technique, with a success rate of over 90% [10,11]. However the technique requires experienced endoscopists who could place the device without delay. Holzinger and colleagues compared the success rate of correct jejunal placement of a self-advancing jejunal tube (Tiger tube) with the gold standard, the endoscopic guided technique. Success rate of correct jejunal placement of the guided self-advancing was significantly lower than the success rate of the endoscopic guided technique [12]. This prospective randomized study did not specify the overall time for the procedure from the call of the endoscopist to the control of correct placement and the beginning of the enteral nutrition.
There are various techniques for the placement of the jejunal feeding tube, among which we should mention the electromagnetic technique, the results of which appear promising in terms of its rapidity, but which demand lengthy training times for medical and nursing staff [13]
A recent retrospective study comparing the self advancing jejunal tube Tiger 2 (T2T) and Cortrak Enteral Access System (C-EAS) showed the T2T was more effective at post pyloric placement on first attempt, because, despite the real-time visualization with the C-EAS system, the success rate is user dependent. This study points the need for additional provider training on using the C-EAS system and interpreting tracings or a dedicated tube insertion team.
In a prospective observational study of Deane et al. colleagues the electromagnetically guided device enabled safely and rapid bedside placement of small intestinal feeding tubes, suggesting the high-level manual dexterity is not necessary for its successful use [14].
Another procedure, the modified Corpak 10-10-10 protocol, consists in the administration of metoclopramide and the insufflations of 500 ml of air into the stomach during the progression of the feeding tube to facilitate the opening of the pyloric sphincter along with control of the ph to confirm its correct placement [15].
Our multicentre observational prospective study aims to assess safety and feasibility of the placement of the self-advancing jejunal feeding tube (Tiger 2) in critically ill patients, describing the feasibility indicators for the use in ICU in term of success rate at the first attempt, length of procedure until correct positioning, timing of starting the enteral feeding and achievement of the caloric need.
Prospective data were collected from 5 mixed medical-surgical, adult intensive care units of 5 Italian hospitals between January 2013 and January 2014. Inclusion criteria were patients aged ≥ 18 years with a diagnosis of acute pancreatitis, abdominal compartment syndrome (postsurgical or traumatic) and severe gastric stasis (≥ 500 ml) at the admission. In the remaining patients the nursing staff placed a gastric feeding tube at admission to the intensive care unit, proceeding then with a serial measurement of gastric stasis.
In the case of persistent gastric stasis ≥ 250 ml, despite the administration of prokinetic drugs (erythromycin or metoclopramide) after 3 days, the medical staff decided that jejunal enteral nutrition was indicated, and trained nursing staff performed the placement of a Tiger 2 self-advancing jejunal feeding tube. Exclusion criteria were oesophageal and gastric varices or strictures, previous major gastro-oesophageal surgery, coagulation disturbances and suspected or documented bowel ischemia.
Each intensive care unit collected the data by compiling a specific record card for each patient. The data record card contained the patient’s characteristics (height, weight, BMI), the morphological aspect (normotype, brachytype or longitype), the entry diagnosis (multiple injuries, sepsis, neurological, respiratory, cardiac, pancreatic disease, abdominal surgery, neurosurgery, i.a.), the scheduled calorie requirement (20-25 Kcal/kg/day), the reason for, and timing of the insertion of the jejunal feeding tube (presence of gastric stasis and/or signs of intolerance of enteral nutrition via the gastric route such as vomiting or increased pancreatic enzymes), presence of mechanical ventilation, presence of peristalsis and its characteristics (torpid or lively), the use, if any, of prokinetic drugs (erythromycin or metoclopramide), progression time (minutes/hours), success (1st, 2nd attempt or use, if any, of endoscopy), the extent of residual gastric stasis (≥ 0 ≤ 250 ml) after the placement, radiological examination (plain abdominal x-rays), time taken to achieve the scheduled calorie requirement (days), side effects (diarrhoea, vomiting) and presence or absence of pain on removing the feeding tube.
The self-advancing jejunal feeding tube (Tiger 2) was inserted by trained nursing staff (previous successful placement at least of 3 feeding catheters) under supervision of a consultant intensivist. For the placement manoeuvre of the Tiger 2 feeding tube, the staff followed the indications summarised in the Appendix. In not-ventilated patients, the manoeuvre was performed together, in some cases, with the administration of a mild sedative according to the department protocol.
The Tiger 2 self-advancing jejunal feeding tube is made of polyurethane, which is an extremely soft and pliable material. Its softness, however, may make it more difficult to advance through the initial portions of the nasal cavities and hypo pharynx. It is possible to use an optional rigid wire to act as a guide as far as the stomach, making the feeding tube stiffer and preventing it from twisting or coiling during advancement. The optional rigid wire was used in those patients in whom entry at the nasal level proved impossible (for example, due to nasal injury), whereas in the remaining cases the jejunal feeding tube was cooled in a refrigerator for approximately one hour before performing the manoeuvre.
The patients were evaluated on the basis of their morphological aspectnormotype, brachytype and longitype (prevalence of the transverse or longitudinal diameter). On the basis of the morphological aspect, the jejunal feeding tube was inserted into the stomach as far as 55 cm in the brachytype subjects, as far as 65 cm in the normotype subjects and as far as 70 cm in the longitype subjects. This procedure was necessary in order to prevent excessive advancement at the gastric level causing twisting or coiling of the device, with the need to withdraw the tube and repeat the manoeuvre. The self-advancing jejunal feeding tube was then left in the stomach for 30 minutes.
The advancement of the self-advancing jejunal feeding tube was 10 cm per hour in the presence of lively peristalsis and 5 cm per hour in the presence of torpid peristalsis until a distance of 100 cm was reached. Each advance was verified by means of the insufflations of air or water and stethoscope auscultation. On reaching a distance of 75-80 cm (variable on the basis of the morphological aspect), - this being a landmark corresponding to the external projection of the pyloric sphincter (right hypochondrium), - the patient was given a bolus administration of the prokinetic drug (erythromycin 250 mg, metoclopramide 10 mg i.v., according to the department protocol). The advancement was perceived from the tactile point of view as a sensation of slight resistance. Therefore, at this level, the feeding tube was subjected to a rotatory movement in order to facilitate its passage through the pylorus.
The patients were then placed in the right lateral decubitus position in order to facilitate the passage of the feeding tube by gravity via and beyond the pyloric sphincter. This manoeuvre was not adopted in patients with active acute neurosurgical pathology or with possible problems in controlling intracranial pressure, in patients with pelvic or femoral fractures, in whom this position is precluded, and in patients with active respiratory problems affecting the ipsilateral lung. The remaining advancement up to 100 cm was done according to protocol. The correct positioning of the jejunal feeding tube (penetration beyond the pyloric sphincter) was checked at the end of the procedure by performing an abdominal x-ray in anteroposterior projection. The radiograph was independently verified by a radiologist. Enteral therapy was initiated once correct placement of the feeding tube was verified. As regards the nasogastric tube positioned at admission to the department, this was left in place in the presence of severe gastric stasis and removed on normalization of the gastric stasis values. The study procedures followed were in accordance with the ethical standards of the responsible institutional committee on human experimentation.
In 89 patients placement of the self-advancing jejunal feeding tube was achieved at the first attempt (82%). In 7 cases (6%) it was placed at the second attempt. In no case was the feeding tube accidentally placed in the airways. The median time for placement of the T2 self-advancing jejunal feeding tube according to protocol was 4 hours.
The tube placement success rate, regardless of the number of attempts, was greater in non-neurosurgical patients (Graph). Most of the critical patients examined consisted of patients suffering from primary or posttraumatic acute pancreatitis, whereas the remainder of the population consisted of neurosurgical patients, deeply analgosedated with opioids for medium-to-long periods, and of polytraumatized patients. A minority of patients presented respiratory disease, undergoing mechanical ventilation and periods of prolonged respiratory weaning. Demographic and clinical characteristics of the patients are shown in (Table 1).
On verifying correct placement, it was possible to initiate enteral therapy and achieve the scheduled caloric requirement in a mean time period of 48 hours. None of the patients receiving nutrition via the jejunal route presented side effects such as diarrhoea or vomiting. The median time of permanence of the self-advancing jejunal feeding tube in situ was 14 days. The jejunal feeding tube removal manoeuvre, performed slowly and imparting a rotatory movement to the tube, was well tolerated in 90% of patients. Only 2 non-intubated patients complained of stabbing pain in the chest during removal of the tube.
This multicentre observational prospective study has shown that placement of a Tiger 2 self-advancing jejunal feeding tube appears to be effective and relatively rapid in critical patients suffering from pancreatic and abdominal disease and in patients with persistent gastric stasis despite the administration of prokinetic drugs, adopting a placement technique that exploits a number of considerations regarding the anatomy of the gastrointestinal tract and the pharmacological properties of prokinetic drugs. Anatomically, the pyloric sphincter constitutes the critical point for the progression of any device.
The opioids and vasopressor drugs commonly used in the treatment of critical patients produce a contraction at the level of the pyloric sphincter, making the passage of any device a difficult matter. It is therefore fundamental to adopt manoeuvres that facilitate sphincter relaxation, even only temporarily, and in the first place the timing of the use of prokinetic drugs. The pharmacokinetics of these drugs means that the maximum action after bolus administration occurs within 10 to 15 minutes, a phenomenon that we exploited to facilitate the passage through the pyloric sphincter.

Table 1: Demographic and clinical characteristics of the 109 study patients
Metoclopramide is a dopaminergic blocker, commonly used to treat nausea and vomiting, to facilitate gastric emptying [16]. Erythromycin is used in the treatment of infections and as prokinetic agent enhances gastric motility. However, the results of research about the use of prokinetic agents are controversial. Lai and colleagues achieved a success rate of 57% using a spiral tube compared with 0% using a straight tube in patients with abnormal gastric emptying who received prior administration of 10 mg metoclopramide [17]. A recent prospective, multicentre, open-label, randomized, controlled clinical trial suggests that metoclopramide may facilitate postpyloric placement of spiral nasojejunal tubes in critically ill patients [18].
On reaching and entering the pyloric sphincter, the T2 self-advancing jejunal feeding tube is facilitated in its progression by placing the patient in the right lateral decubitus position which propels the tube by gravity towards the duodenal C loop. The cilia-like flaps, which are a peculiar characteristic of the T2 self-advancing jejunal feeding tube, not only permit the advancement at this point but also prevent displacement of the tube and its recession into the stomach.
The adoption of these techniques has made the placement of the device a relatively rapid process and has permitted early initiation of enteral nutrition. In the intensive care units recruited into this study, the placement of the jejunal feeding tube is usually performed by a gastroenterologist who, through the use of endoscopy, enjoys a direct view of the pyloric sphincter. It is, however, not always possible for the intensive care staff to avail themselves of the aid of the endoscopy service round the clock, thus further delaying the start of enteral nutrition. Endoscopy, which as a positioning technique under direct view has a maximum success rate of 92% [19], sometimes turns out to be a failure for the endoscopist as a result of the endoscope extraction manoeuvre, inasmuch as the jejunal feeding tube is also dragged backwards with it, resulting in a further delay for the intensive care staff in initiating enteral nutrition.
The indication for placement of the jejunal feeding tube is reached following a protocol evaluating the persistence of gastric stasis and/or signs of intolerance of enteral nutrition via the gastric route. Cooperation between the medical team and nursing staff in assessing these phenomena proved to be of fundamental importance for the purposes of feeding critical patients with the most appropriate timing and methods. The device placement manoeuvre performed by trained nursing staff and supervised by intensive care physicians appears to be simple, safe and rapid.
The patient population recruited into our multicentre, observational, prospective study came from 5 Italian mixed medical-surgical, adult intensive care units. It appears clear that the kinds of patients can basically be divided into two groups, the first comprising patients suffering from acute pancreatic disease and abdominal compartment syndrome, and the second consisting of patients suffering from neurosurgical disease.
As regards the first group, the problems of gastric stasis and intolerance of enteral nutrition are strictly related to the abdominal disease in progress. As far as this category is concerned, the several comparative studies published in recent years have established substantial equi-efficacy of gastric and jejunal approaches for achieving the patient’s caloric requirement, it being the case, however, that in clinical practice these patients can hardly be fed in the stomach [20].
As regards the group of neurosurgical patients, it is well known that cranial pathology induces the phenomenon of gastro paresis via a neuronal cascade; accentuated and protracted by the sedation these patients normally require [21]. Abnormalities in the gastrointestinal transit may be observed in brain injury patients, including decreased peristalsis or a reduction of the antral contraction [22]. This effect can be occasional or persistent during the first days after injury or can match acute episodes of intracranial hypertension. In fact, patients with increased intracranial hypertension have as much as an 80% increase in the incidence of elevated gastric residuals [23]. The precise impact of these challenges related to neurocritical illness on nutrition provision has not been ascertained, but many of these factors may limit the extent of nutrition provided in the first week of illness [24].
The transpyloric approach in patients with traumatic brain injuries is known to increase the calorie supply and markedly reduce the incidence of infections and the number of days spent in intensive care [25]. Moreover the prospective, open-label, randomized study of AcostaEscribano and colleagues concluded that brain injury patients fed early through a transpyloric tube and treated with a set of measures to reduce the incidence of bronchoaspiration, including strict control of the head position, early use of prokinetic drugs, and strict application of feeding guidelines had lesser incidence of late pneumonia [26].
The published studies focussing on the endoscopic placement of the jejunal feeding tube emphasize the difficulty encountered in performing the endoscopy and the delay in initiating enteral nutrition in these highly catabolic patients [27]. The need to focus attention on this group of patients who could potentially benefit more from this approach is therefore obvious, as emerges also from our multicentre observational prospective study. In the light of our results that demonstrate that the success rate of feeding tube placement was greater in non-neurosurgical patients, regardless of the number of attempts, we have reflected on the data and come to the conclusion that the rapidity of placement of the T2 self-advancing jejunal feeding tube is strictly related to correct timing of compliance with the protocol (Figure 1).
Identification of neurosurgical patients, deeply analgosedated with opioids, predictably with severe gastric stasis and/or signs of intolerance of nutrition via the gastric route, and inclusion of this element in the protocol, without delay, increased the success rate of placement of the device in a shorter time. Other prospective studies need to be conducted regarding manoeuvres that further reduce the placement time of the self-advancing jejunal feeding tube, paying particular attention to the need to comply fully with a protocol that assesses the tolerance of enteral nutrition and permits the early identification of patients requiring a jejunal approach.

Figure 1: Success rate and progression time of Tiger 2 self-advancing jejuna tube in neurosurgical patients and in other ICU patients.
Download Provisional PDF Here
Aritcle Type: Research Article
Citation: Zatelli M, Carletti A, Acqua GD, Falchi R, Cima M, et al. (2015) Self-Advancing Jejunal Feeding Tube in Critically Ill Patients: A Feasibility Study. J Gastric Disord Ther Volume 1(1): doi http:// dx.doi.org/10.16966/2381-8689.104
Copyright: © 2015 Zatelli M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Publication history:
All Sci Forschen Journals are Open Access