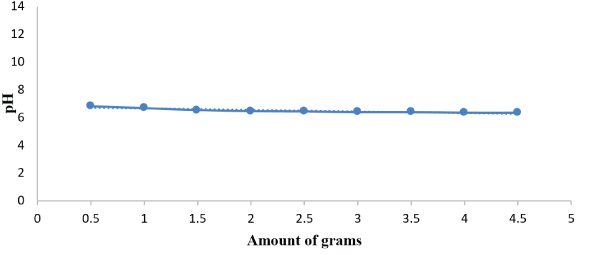
Figure 1: pH values of dried sewage sludge.


Tarek S Metwaly1* E.H.El-Mossalamy2 Gamal O El-Sayed3 Eman Tahawy4 Nafiesa M Elgendy5
1Egyptian Water and Wastewater Regulatory Agency, Masraweya District, 5th Community, New Cairo city, Egypt*Corresponding author: Tarek S Metwaly, Egyptian Water and Wastewater Regulatory Agency, Masraweya District, 5th Community, New Cairo city, Egypt, Tel: +20 01013920189; E-mail: tarekgood100@yahoo.com
Malathion is a dangerous and harmful substance because of its toxicity for humans, animals and aquatic life. Dewatered sewage sludge collected from the disposal area of sludge treatment (drying beds stage) that obtained from wastewater treatment plant in Daqhlia Governorate was thermally treated at three different temperatures 100, 375 and 500°C (which called SLG, SLG1and SLG2 respectively and used to remove malathion from aqueous solution. Experiments were performed at different doses (0.5-1-1.5-2-2.5-3)g of SLG, SLG1 and SLG2 and were shaked at different pH values (2-4-6-8-10-12) at different temperatures. The effect of operational indicators on removal rates has been studied, tested, improved and optimized. The optimum dosage of biomass dried sludge to remove 36.765µg/L of malathion from aqueous solution was 1g/100 mL and the optimum contact time was 30 minutes as the removal efficiency reached to 98%. Adsorption isotherm experiments were performed and showed that the Langmuir model fitted the experimental data well as compared to other models and the malathion adsorption onto SLG followed pseudo second-order model, through which the results obtained to be consistent with the practical results that were implemented.
Malathion; Dried sludge; Adsorption
People who live near areas where malathion is sprayed are exposed to many dangers through skin contact with contaminated plants and soil, inhalation of droplets formed during application, and ingestion of residues in food or water. Malathion is an organic substance and is used as a highly toxic insecticide in various fields to kill ants, mites, insects, mosquitoes, etc. by inhibiting cholinesterase. Malathion is rapidly absorbed by all through the gastrointestinal tract, skin, mucous membranes, and lungs. Malathion causes headache, dizziness, headache, nausea and vomiting, affects the nervous system and is toxic to birds, fish, honeybees and other aquatic invertebrates [1].
Malathion solution in its pure form is colourless and has a yellowishbrown color and is characterized by the smell of garlic when added as a portion of the solution in its pure form [2]. Malathion (C10H19O6 PS2) is a phosphorous pesticide and one of the organic compounds used to control insects and moths on a large scale in agriculture, orchards, house flies and mosquitoes [3].
Malathion is emitted to the environment by spraying on crops or maintaining public health in urban or non-residential areas. But malathion may be spread to other sites by rain or wind, and the malathion compound is broken down in the environment within a few weeks as it does not stick to the soil. It is rapidly degraded in soil and water by bacteria and movement into groundwater is not significant.
If malathion is present on dry soil or on man-made surfaces, The movement of malathion in groundwater is not significant. When it is on a dry soil or surface, Malathion does not decompose quickly, but it decomposes in other aqueous media. Malathion is rapidly metabolized or purified and has no potential for biomagnification in the food chain [2].
Malathion is absorbed biotransformed and excreted in urine and faeces largely as monocarboxylic acid and dicarboxylic acid [4]. Malathion is toxic to birds and has a wide range of toxic effects on aquatic invertebrates, fish, and honeybees [5]. Almost all of the observed effects of malathion are due to its active metabolite on the nervous system. Malathion is known to be moderately toxic to birds, has a wide range of toxicity in fish and other aquatic invertebrates, and is highly toxic to honey bees [5].
Several studies were conducted on rats to study the ability of malathion to cause cancer, but there were mixed results in the April 80, 2000, toxicological and hazardous studies of agricultural chemicals. The US Environmental Protection Agency has suggested that malathion is carcinogenic, but the reasons are insufficient to assess the potential for malathion to cause human cancer through exposure routes [6].
The removal of malathion using drumstick peel powder and bagasse fly ash was affected by the adsorbent dose, initial Malathion concentration, initial pH, contact time. Removal of malathion from water has been taken up by jar test apparatus technique using polyaluminum chloride and polyelectrolyte.
The optimizing conditions for successfully reducing TSS, BOD, COD. The adsorption properties of malathion and egg shell material was thermally treated [7].
Among the most unconventional adsorbents used is fly ash which is distinguished from the others, in that it is waste of using thermal power plants and causes severe damage to the environment and industries [8].
In Tunisia, with the progress and development of sewage treatment plant projects, the quantities of sewage sludge generated from treatment plants have increased and many studies [9-11].
It has been studied that the degradation of malathion under various conditions, including ultrasonic, ultraviolet (UV), combined US/UV, UV/ZnO and the effect of operational parameters was studied and necessary analysis were performed [12]. Some methods have been proposed to treat malathion, such as chemical oxidation processes, which have received attention due to high efficiency in chemical detoxification, efficiency in mineralization and detoxification [13]. Researchers using sodium alginate/biosilicate/magnetite (SABM) nanocomposite for removal of malathion [14].
The problem of sludge is summarized in the increasing requirements of government institutions in seeking to achieve better standards of environmental quality, which was manifested in improving the management of sewage services, and it is expected that the number of sewage treatment plants will increase in the coming years, which will be followed by an increase in the quantities of sludge generated from these plants, which face a problem in getting rid of it periodically, which causes problems in operation, public health and the environment. Especially in the delta areas of Egypt, which contain many treatment plants and production large quantities of sludge, in addition to the presence of agricultural drains that contain many pollutants, the most important of which is pesticide residues. Therefore, it was necessary to study the use of the sludge resulting from these plants to use it in treating water pollutants from pesticides used in agriculture, such as malathion.
This research paper aims to study the possibility of using dewatered sewage sludge which has been collected from the final disposal area of sludge treatment which called (drying beds) and it was ground and thermally treated at three different temperatures 100,375 and 500°C which called SLG, SLG1and SLG2 respectively
Malathion [s-1,2-bis (ethoxycarbonyl) ethy-o,o-dimethyl phosphor-dithioate] with molecular formula C10H19O6PS2 and formula weight of 330g/mol was obtained from ADWIA, Egypt. Organic solvents used in extraction method were pesticide grade iso-octane and hexane. Water solutions used were deionized water produced in the laboratory. The solubility in water was found to be 145mg/L. A solution of malathion was prepared with concentrations of 36.765 and this weight was stabilized on the scale, and a stock was made to be used in the experiment.
The dried activated sludge biomass was obtained by collecting dried activated sludge from the municipal waste water treatment plant. The sludge was dried in an oven at 100o C for 2 hours and dry sludge was thermally treated at three different temperatures 100, 375 and 500°C (which called SLG, SLG1and SLG2 respectively and it can be regenerated by washing of tap water or distilled water after using in malathion removal.
pH meter (ELISCO L 1612).
The XRF analysis was carried out for powder (<74µm) samples using X-Ray fluorescence equipment PW2404 with six analysis crystals. Crystals (LIF-200), (LIF-220) were used for estimating Ca, Fe, K, Ti, Mn and other trace elements from Nickel to Uranium.
The IR spectra were carried out using Mattson 5000 FIIR spectrometer in the rang 4000-400cm-1 for a sample of 2-3mg diluted with 300 mg KBr.
The Scanning Electron Microscopy (SEM) photographs model Philips XL30 attached with EDX Unit, with accelerating voltage 30 K.V., magnification 10x up to 400.000x and resolution for W. (3.5nm).
Gas chromatography (GC mass- clarus 500) Organic solvents used for extraction were pesticide grade iso-octane and hexane. Water solutions were made with distilled/deionized water prepared in the laboratory to estimate malathion.
Kinetic adsorption experiments were carried out to assess the effect of the adsorption process on time and to determine the rate of removal of malathion, and the effect of kinetic adsorption on pH and temperature. The operations that were performed in the laboratories are described as follows:
1) Preparing 100 ml of a solution containing a certain amount of malathion.
2) Stirring these solutions on a mechanical stirrer at a speed of 300rpm.
3) Adjust the pH value of the solution to the desired value.
4) Add the amount of dried activated sludge to the solution.
5) Analysis of residual malathion in samples.
Adding different weights of adsorbents i.e. 0.5, 1, 1.5.2, 2.5, and 3g of SLG, SLG1, and SLG2 into 100ml of 36,765µg/L in malathion aqueous solution at pH 6 for 30 min and the mixture shaken using a Vibromatic-384 shaker at 300 rpm and 25°C.
The effect of conditioning time on the adsorption process of malathion was investigated by dry sludge SLG, SLG1 and SLG2. Portions of dry sludge were placed in flasks containing 100mL of 300ppm malathion aqueous solution 36,765µg/L at pH 6, In this research paper, the contact time for removal of malathion was implemented in a relatively short period of time range of 5–30 min in order to determine the effect of contact time on the removal of malathion.
We have studied the effect of changes in pH on adsorption of malathion on the adsorption factors of SLG, SLG1 and SLG2 respectively at pH ranging from 2 to 12 was tested.
Adsorption isotherm has given idea about the feasibility of an adsorbate–adsorbent system. In this work Langmuir and Freundlich were tested for describing the experimental results, Adsorption isotherm experiments were performed by adding 1gm of SLG, SLG1 and SLG2 to 36.6µg/L in 100ml of malathion solution in a glass bottle.
pH: The pH results for the sludge samples ranged between 6.3 and 6.8. The pH controls the bioavailability of minerals, especially those minerals which are largely present in a variable form as shown in figure 1. The pH of sewage sludge changes between acidic properties to neutral and alkaline properties depending on the degree of treatment and application conditions sludge. Crops are known to grow when the soil pH is between 6 and 7 because nutrients are more readily available around pH 6, and since the pH values of most analyzed sewage samples fall within the required range (between 6 and 7). Further stabilization may not be necessary for application on non-alkaline and non-acidic soils [15]. The characterization of sewage sludge to know its characteristics before use is important and this is useful while applying sewage sludge to check the risks of its use which is illustrated in figure 1.

Figure 1: pH values of dried sewage sludge.
Analysis of dried sludge by TGA: The thermal analysis was carried out using Schimadzu DTA-50H and TGA-50H. Each powdered sample is heated by 20°C/min up to 800°C with α-Al2O3 as references material. The presented analysis shows the mineral constitutes of the studied sample and the characteristic thermal behavior of each mineral. The combining Pattern of Sludge and water have four ways is shown in figure 2, the first is free water, which contains in the microcell Intercellular not combining close with cell, so it is volatile and its volatilization temperature from 20°C-40°C, the second is combined water by physical adsorption, and its volatilization temperature is from 40°C-110°C, the third is combined water by chemical adsorption, and its volatilization temperature is from 200°C to 400°C, the fourth Four is bound water by chemical bond combination, and its volatilization temperature is from 600°C to 800°C.
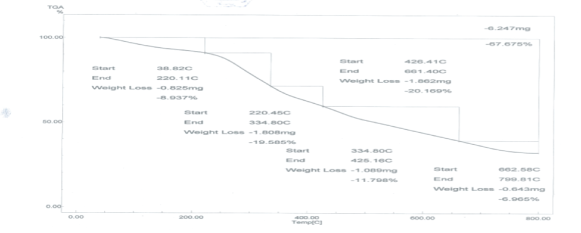
Figure 2: Thermal Analysis of Dry sludge from plant using Thermogravimetric Analyzer (TGA).
In this study, the particle sample is dried at temperatures of 103°C, free water in the particle sample is completely removed. Therefore, water of weight loss before 105°C can be considered as a combination of physical adsorption in thermogravimetric date [16].
The effect of the amounts of adsorbents on the efficiency of malathion removal was studied by adding different weights of adsorbents in malathion aqueous solution at pH 6 for 30min, in order to obtain the optimal biomass concentration corresponding to the maximum binding capacity. It is clear from figure 3 that the increase in the amounts of SLG and SLG2 led to an increase in the surface area with more adsorbent sites for adsorption to occur [17].
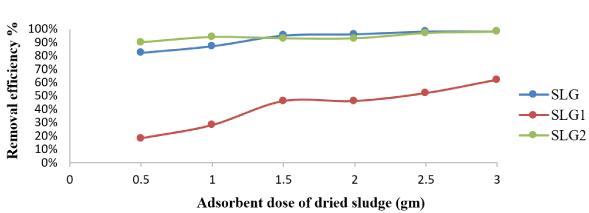
Figure 3: Effect of adsorbent dosage of SLG, SLG1 and SLG2 on adsorption of malathion ions. [adsorbate initial concentration: 36.765 μg/ L; contact time: 30 min; pH 6; agitation speed: 300 rpm].
Some studies confirmed the efficiency of removing organophosphorus pesticides increases with increasing contact time [18] and initially the results showed that, adsorption rate of malathion onto SLG, SLG1 and SLG2 is found to be rapid, because of the increasing of adsorption sites, On the other hand, the uptake of malathion by dry sludge in its various forms such as SLG, SLG1 and SLG2 was investigated shown in figure 4. This adsorption is attributed to the strong molecular interaction between the malathion molecules and the dry sludge, causing them to aggregate in the absorbent layer [19].
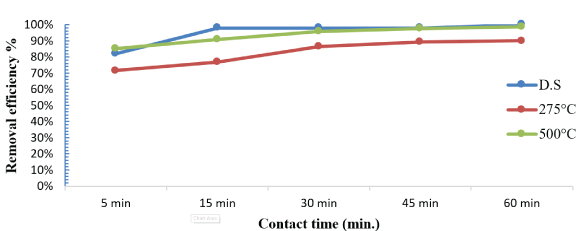
Figure 4: Effect of contact time on adsorption of malathion ions. [adsorbate initial concentration: 36.765 μg/ L; amount of doses :1g; 30 min; pH 6; agitation speed: 300 rpm].
The laboratory results showed that the absorption of malathion by the dried sludge increases from 81% to 98% with an increase in temperature from 100 to 500 at pH 6 with an initial concentration of 36,765μg/L shown in figure 5. It is known that the increase in temperature helps to overcome the energy barrier and adhesion to the surface, and there are some other possibilities represented in the presence of additional adsorption sites on the surface of the dry sludge resulting from the disintegration of the surface of the dry sludge at a temperature higher than the surrounding temperature, which increases the malathion absorption indicating that the adsorption is endothermic, On the other hand, malathion has low thermal stability and rapidly degrades with increasing temperature, as a result, the temperature in all practical tests was fixed at the normal laboratory temperature.
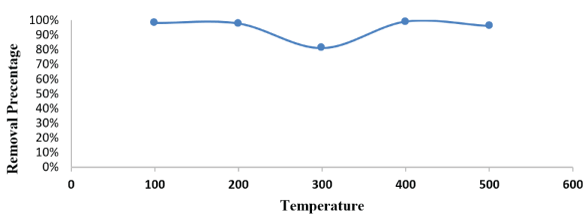
Figure 5: Effect of temperature on adsorption of malathion solutions. [adsorbate initial concentration: 36.765 μg/ L; amount of doses :1g; 30 min; contact time :30 min.; pH 6; agitation speed: 300 rpm].
In general, increasing the concentration of pesticides in solutions will reduce the removal efficiency [20]. The data presented in figure 6 showed the changing on initial malathion concentration from 5µg/L to 100µg/L. The percent removal decreased from 98% to 89%, 96% to 59%, and 98% to 79% for SLG, SLG1 and SLG2 respectively in figure 6. In general, the concentration of pesticides is increasing in the samples which will reduce the removal efficiency [21]. It is evident from the figure 6. that, the adsorption increases with increasing time. Although percent removal with higher concentration is less, the amount of malathion adsorbed on dry sludge SLG, SLG1 and SLG2 is higher than that at lower initial concentrations.
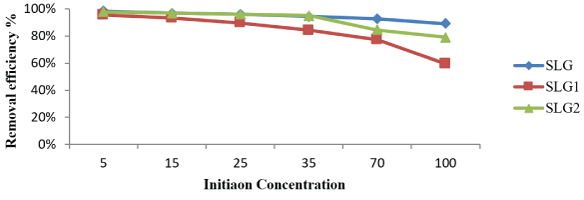
Figure 6: Effect of initial malathion liquid solution concentration. [Amount of Doses: 1g; 30 min; contact time: 30 min.; pH 6; agitation speed: 300 rpm].
It was also observed that, the initial concentration of malathion is increased and it is needed to achieve the equilibrium at an initial malathion concentration of 100µg/L, the equilibrium time is 30min.
Since the surface charge of sludge adsorbents can be modified by changes in pH values in aqueous solutions between 2-12, When the pH value decreases, the malathion removal efficiency decreases, as the percentage of removal efficiency increases dramatically when the high pH values rise as a result of the positive interaction between the dose of malathion and the excellent and it is shown in the figure 7. This interaction is related to the electrostatic interaction to increase the values of pH [17].
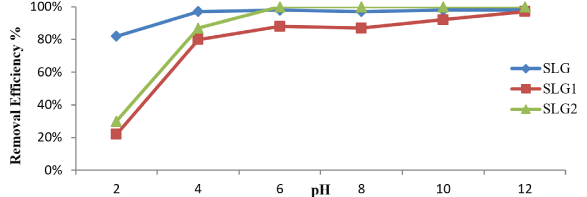
Figure 7: Effect of pH on removal of malathion ions. [amount of doses :1g; adsorbate initial concentration: 36.765 μg/ L; contact time :30 min.; agitation speed: 300 rpm].
Langmuir study: Langmuir isotherm in the system indicates on mono layer malathion coverage on the outer surface of the dry sludge. From the linear plot of an experimental sorption data 1/qe vs. 1/Ce for the malathion concentrations from 5 to 100µg/L are given in the table 1. The isotherm indicators RL, qmax and R2 are determined at fixed pH 6. The malathion uptake was calculated by determining the residual malathion concentration in the supernatant according to the above method and according to the following equation:
Where qe (mmol/g) is the uptake of malathion at equilibrium, C0 and Ce (mmol/L) are the concentration of malathion at initial and equilibrium, respectively, m(g) is the mass of the used dry sludge (SLG). All these values are shown in table 1. We note that, RL indicates the affinity of the adsorbents to the adsorbate and qm indicates the maximum adsorption capacity. As figures 8-10 indicates, for given values of the initial concentration, pH and average particle size of the adsorbent, the equilibrium concentration of Ce, of the adsorbent in solution is higher at lower temperature and decreases with increasing temperature, meaning that it favors adsorption at higher temperature. The applicability of the Langmer isotherms in the current system indicates that a monolayer of malathion is covered on the outer surfaces of the absorbent.
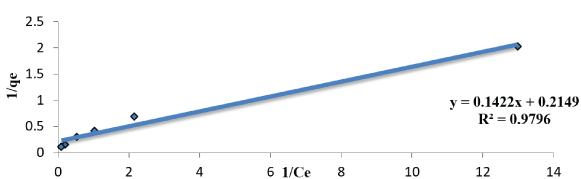
Figure 8: Linearized Langmuir adsorption Isotherms of malathion solution with biomass SLG (pH; 6; amount of doses; 1gm agitation rate 300 rpm.
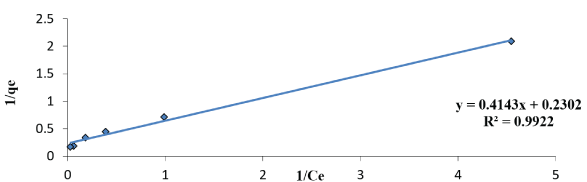
Figure 9: Linearized Langmuir adsorption Isotherms of malathion solution biomass SLG1 (pH; 6; amount of doses; 1gm agitation rate 300 rpm).
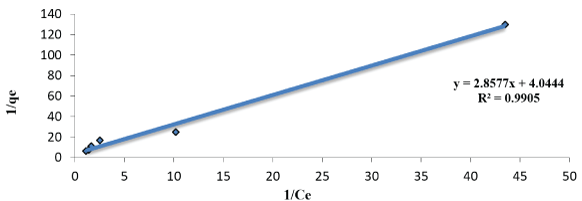
Figure 10: Linearized Langmuir adsorption Isotherms of malathion solution with biomass SLG2 (pH; 6; amount of doses; 1gm agitation rate 300 rpm).
| Langmuir Model | ||||
| Kl | qmax | RL | R2 | |
| (SLG) | 0.14 | 4.6 | 0.15 | 0.979 |
| (SLG1) | 1.7 | 4.3 | 0.014 | 0.992 |
| (SLG2) | 1.4 | 6.25 | 0.01 | 0.990 |
Table 1: Langmuir model parameters for adsorption of malathion onto SLG, SLG1 and SLG2 respectively.
Spectra of FT-IR Spectrum showed that, the groups of –NH and OH and CO-O-CO at 33823, 3434 and 1043Cm-1, these groups play an extremely important role in binding of malathion ions and illustrated in figure 11.
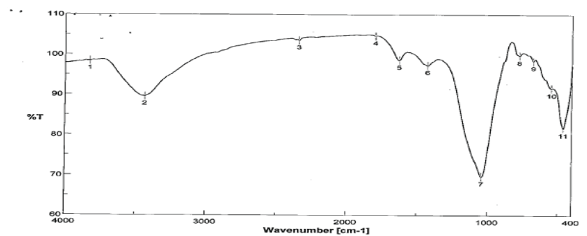
Figure 11: Infrared Spectra of SLG2 after malathion sorption.
We selected two different kinetic models, pseudo first order and pseudo second order are given in the figure 12 and 13. From the data obtained in table 2, The values of qe and R2 calculated from pseudo second order kinetic model are agreeable because the value of R2 > 0.9 and qe in pseudo second order kinetic model is better for predicting the kinetic (3.57) than pseudo first order kinetic model (0.0716).
Elovich model: The most interesting model to describe the dry sludge adsorption is Elovich model which assume that, the rates may decrease with time due to an increase in surface coverage [22]. One of the most useful models for studies of such as chemisorption is the Elovich equation (2)
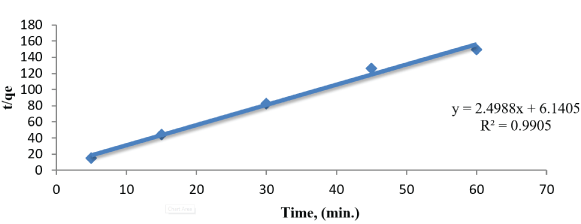
Figure 12: Plot of the pseudo-first-order adsorption model malathion onto 1g of SLG.
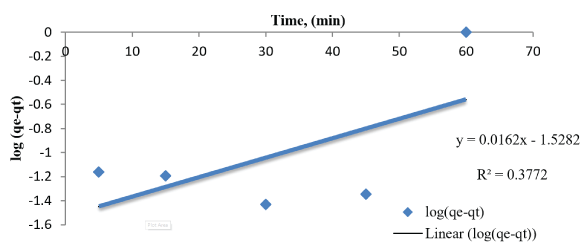
Figure 13: Plot of the pseudo-second-order adsorption model malathion onto SLG.
| First-order-model | Second-order-model | ||||
| R2 | K1 | qe mg-1min-1 |
R2 | K2 mg g-1min-1. |
qe mgg-1min-1 |
| 0.3772 | 0.115 | 0.0716 | 0.99 | 1.168 | 3.573 |
Table 2: Kinetic parameters of adsorption of malathion onto SLG.
q=1/blnab+1/blnt .....(2)
Where, ‘a’ and ‘b’ are constants. The constant ‘a’ is considered as the initial adsorption rate (mg/g min) and ‘b’ is related to the extent of surface coverage and activation energy for adsorptions (g/mg) is given in the figure 14.
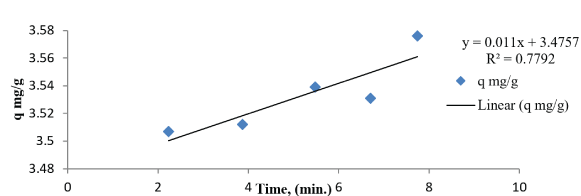
Figure 14: Elovich model of malathion onto SLG.
Laboratory experiments were performed on the adsorption process to remove malathion from aqueous solutions. In this study, dry sludge after thermally treated at three different temperatures 100°C, 375°C and 500°C which called SLG, SLG1and SLG2 respectively. Application of SLG, SLG1 and SLG2 serves two purposes. Since the sludge currently in treatment plants is solid waste and causes problems in operation as a result of not being disposed of periodically, and secondly, contributing to the removal of malathion (%).
The synthetic waste water by using malathion solution SLG, SLG1 and SLG2 was then filtrated with sample of water to test on gas chromatography in laboratory.
Adsorption isotherm has given idea about the feasibility of an adsorbate–adsorbent system. In this work Langmuir was examined for describing the tests results and fitted the experimental data. Adsorption isotherm experiments were performed by adding 1gm of SLG, SLG1 and SLG2 to 36.3µg/L, and the optimizing conditions at pH 6 and sample volume of 100ml. Due to the groups of –NH and OH and CO-O-CO at 33823, 3434 and 1043Cm-1 and the groups play an important role in binding of malathion ions.
By optimizing the kinetic of adsorption process, results showed that pseudo second-order model provides a good acceleration for the description of the mechanism of sorption of malathion in contrast of first -order model.
Laboratory experiments have shown that sewage treatment plant sludge ash can be used as an alternative, and inexpensive, active ingredient for removing a large amount of toxic pesticide residues from water.
The simplicity of the method of use in adsorption processes at different temperatures, especially those that were burned at 500°C to remove pesticide residues from aqueous solutions at high percentages, is considered a useful matter from an economic and environmental point of view and serves the field of green economy in maximizing the utilization of waste or converting it into valuable and environmentally friendly materials. It can be used well in improving water quality.
SLG is drying sludge heated at 100°C; SLG1 is drying sludge heated at 275°C; SLG2 is drying sludge heated at 500°C; XRD is defined as X-Ray diffraction; TGA is defined as thermal gravimetric analysis.
Download Provisional PDF Here
Article Type: RESEARCH ARTICLE
Citation: Metwaly TS, El-Mossalamy EH, El-Sayed GO, Tahawy E, Elgendy NM (2022) Removal of Malathion from Aqueous Solutions Using Dried Sludge Produced from Municipal Wastewater Treatment Plants in Daqhlia Government in Egypt. J Envi Toxicol Stud 4(1): dx.doi. org/10.16966/2576-6430.131
Copyright: © 2022 Metwaly TS, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Publication history:
All Sci Forschen Journals are Open Access