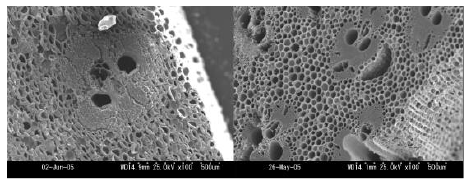
Figure 1: Scanning electron micrographs for the cross sections of stem for bamboo: left, raw structure; right, carbonized at 800 centigrade

Yutaka Kawahara1* Hiroyuki Wakizaka2 Kazuyoshi Yamamoto3 Noboru Ishibashi3
1Division of Environmental Engineering Science, Gunma University, 1-5-1, Tenjin-cho, Kiryu 376-8515, Japan*Corresponding author: Yutaka Kawahara, Division of Environmental Engineering Science, Gunma University, 1-5-1, Tenjin-cho, Kiryu 376-8515, Japan, Tel/ Fax: +81-277-30-1491; E-mail:kawahara@gunma-u.ac.jp
The investigations were made on the carbonization and the activation behaviors for bamboo, and the applicability of bamboo to the production of high functional activated carbons was discussed. Carbon dioxide was used as an activating agent. In the activation process, it was found that the potassium fractions mainly settled in the intercellular regions work as a catalyst and will accelerate the thermal decomposition of carbon. Therefore, when the activation reaction propagated into the intercellular regions, the activation reaction was suddenly accelerated; this makes the pore size distribution of obtained activated carbon featureless. In order to obtain the activated carbon with sharp pore size distribution suitable to remove musty and earthy off-odors from drinking water or harmful volatile organic compounds (VOCs) from ambient air, it is important to control the amount of potassium fractions in the charcoals.
Activated carbon; Bamboo; Micro porosity; Smell; Drinking water
Pitch and coke, the heavy fractions of petroleum and coal, respectively, are the two major starting materials for obtaining various carbons, such as carbon blacks, carbon fibers, and activated carbons (AC). Their carbonization behavior and the properties of the resulting carbons have been extensively studied. However, such carbon resources are not sustainable because of their limited reserves and the rising price of oil. Instead of fossil fuels, several alternative starting materials have been proposed, such as coffee lees [1], triacetylcellulose waste [2], and oak woods [3], to produce AC.
ACs are very useful materials for the elimination of musty off-odours from drinking water as well as the removal of harmful volatile organic compounds (VOCs) e.g. benzene, toluene, and xylene. In the case of Japan, for example, a new law dealing with the quality of drinking water has been issued in 2003 in which the concentrations for two chemicals, 2-methylisoborneol (2-MIB) and geosmin, causing musty and earthy offodors are regulated below 10 ng/ L.
According to the nomenclature of the International Union of Pure and Applied Chemistry (IUPAC), the pores were morphologically classified into three types depending on the mean pore diameter, d, i.e. micro-pore (d<2 nm), meso-pore (2 <d<50 nm), and macro-pore (d >50 nm). For the adsorption of globular shaped molecules with large sizes such as 2-MIB, geosmin, the AC rich in meso-pore will be advantageous [1].
Woody biomass is often used as a starting material for the production of AC. The xylem tissues in woody biomass are strengthened by the development of lignocelluloses structure. Carbons derived through thermal decomposition of natural products usually showed a texture reflecting the original structure of the starting material such as the cellular structure. In this paper, an attempt to produce high functional ACs from bamboo or reed grass was made and the optimal activation conditions were investigated considering the inherent original tissue structure of starting material.
Bamboo (Phyllostachys heterocycla f. pubescens) harvested in Kyoto prefecture and reed grass (Phragmites communis Trinius) harvested in Ono area of Shiga-cho in Shiga prefecture were used after solar drying. Those stems were cut into rectangular slices being 50 mm length, 5 mm width, and 2-3 mm thickness. Other chemicals used were all laboratory grade purchased from Wako Chemical Co. (Osaka, Japan).
The starting materials of 1.5 to 2 g were heat-treated in an electric furnace (CT-40, Advantec Co., Ltd., Tokyo, Japan) having a uniform temperature zone of about 60 mm. During heat-treatment, nitrogen gas flowed through the furnace at a rate of 1 L min-1 at atmospheric pressure. The samples were heated at a rate of 10 K min-1, kept at 800 centigrade for 15 min and cooled to room temperature.
Before the activation procedure, the whole charcoal obtained by the carbonization was ground into powder and the powder was stirred thoroughly to make its quality uniform. Then the ground samples were heated up to 850-950 centigrade at a heating rate of 10 K min-1 under nitrogen gas flow of 1 L min-1. Then CO2 gas was conducted and the concentration of CO2 was kept at 50%. In the temperature range over than 850 centigrade, it is well known that the activation reaction occurs as follows: C+CO2 = 2CO–172 kJ. As the reaction proceeds, the porous structure is produced.
The N2 gas adsorption capacity was determined using an adsorption measurement instrument (BELSORP-18plus, BEL Inc. Jpn, Toyonaka, Japan). The surface area (S) was determined using the BET-plot [4]. The volume (V) was determined from the amount of N2 adsorbed at a relative pressure of 0.948. The mean pore diameter (D) was calculated as D=4 V/S by assuming that the pores are uniform nonintersecting cylindrical capillaries.
The iodine adsorption capacity was determined with the titration method using 0.1 M sodium thiosulfate [5].
Absorption tests against ammonia, formaldehyde, hydrogen sulfide, and toluene were made in a gas state as follows. Each gas of 100 ppm, 3 L was conducted into a bag in which ground AC sample of 25 mg was already settled. Then the change in concentration of gas was monitored.
The liquid-phase adsorption of geosmin on AC was measured as follows. The AC were ground to powder and passed through a 45 µm sieve. The powders were washed, dried and preserved in a desiccators containing silica gel. An aqueous solution of geosmin with a concentration of 400 ng/ L was prepared. AC powder of 0.2 to 0.5 mg was put into the solution of 50 mL. After the solution was stirred for 1 h, AC powder was filtered off. The supernatant was conducted through the solid phase separation column (Empore SDB-XC, 3M, Tokyo, Japan) at 10 ml/ min. The column was dried and the trapped geosmin was extracted with dichloromethane. The amount of geosmin extracted, which equals the amount of geosmin in the bath after the adsorption test, was determined using GC/MS (QP-5050A, Shimadzu, Kyoto, Japan). Similar adsorption tests were made for 2-MIB.
All the surface measurements were made according to JWWA-K113 (2005) regulated by Japan Water Works Association.
Thermal gravimetric analysis (TG) was made from room temperature to 800 centigrade at a heating rate of 10 Kmin-1 under nitrogen gas flow. Heterogeneous contaminative components were characterized using an X-ray microanalyzer (EDAX, AMETEK Co.,Ltd.)
Figure 1 shows cross sections for the raw and the carbonized stems of bamboo. It is seen that the shape of the raw stem tissue was still retained although a big shrinkage occurred during the carbonization process. This suggests that carbonization reactions have proceeded in a solid state without liquefaction. Therefore it should be taken into account that the carbonization and activation behaviors will differ in every tissue reflecting the difference in chemical composition of inherent original structure.

Figure 1: Scanning electron micrographs for the cross sections of stem for bamboo: left, raw structure; right, carbonized at 800 centigrade
Figure 2 shows a result of thermal gravimetric analysis measured for raw bamboo stems. It is seen that thermal decomposition started higher than ca. 200 centigrade. The thermal decomposition temperature for cellulose is over 240 centigrade, whereas for hemicelluloses ca. 180 centigrade [6]. Thus the thermal decomposition of bamboo was considered to be initiated by the pyrolysis of hemicellulose fractions in its tissue. Hemicellulose is a major fraction and rich in the intercellular regions i.e. primary wall or middle lamella and poor in secondary wall (Figure 3). Therefore, it can be deduced that the thermal decomposition of bamboo has started in the intercellular regions firstly, and then propagated to secondary walls.
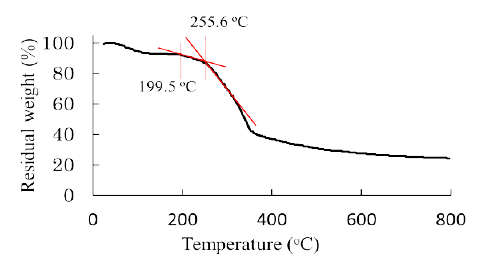
Figure 2: TG curve for the raw bamboo
Figure 3 is a schematic representation for the activation process. The carbonized bamboo still retains its skeleton. Thus, activation gases will diffuse mainly through vascular bundles of bamboo, and then reach each cell and attack secondary wall from lumen side. Then the activation reaction starts from the internal (lumen side of each cell) as well as external surface of sample. Therefore, the nanoporous structure will be constructed firstly in S regions, and then propagate to inner P and M portions.
Table 1 is a list for the surface properties of ACs produced from bamboo at the activation temperature range of 850 to 950 keeping the reaction time for 30 min and CO2 concentration at 50%. It is seen that 900 centigrade was optimal for the activation because the AC with BET surface area almost reaching 1,000 m 2 g-1 could be obtained with securing its yield over 60%.
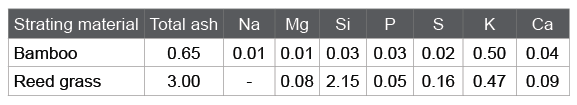
Table 1: Yield and surface properties of bamboo-based ACs activated at various temperatures keeping the reaction time for 30 min and CO2 concentration at 50%.
In figure 4, the time dependence of BET surface area or yield of AC is shown. As the activation time increased, the yield monotonously decreased because of the gasification reaction with CO2 whereas the BET surface area showed a maximum, suggesting the preferable nanoporous structure could be produced at the activation time of 45 min. Such nanoporous structure, however, was destroyed through the random gasification reaction with CO2 at longer activation treatment.
In the activation process, it is known that alkali metal compounds can work as a catalyst for the gasification reaction [7]. Table 2 is a list of the mineral fractions in bamboo- or reed grass-based charcoal obtained at 800 centigrade. Both charcoals contained similar amounts of alkali metal compounds. However, the total ash was much larger for the reed grassbased charcoal due to the siliceous compounds.
The settlement of alkali metals is depending on the botanical metabolism. As for K and Na, these metals are mainly trapped by pectin compounds that are a major component of the primary wall. When the activation reaction propagates into primary wall, the influence of alkali metal compounds on the gasification reaction should be taken into account. In order to investigate the influence of potassium content on the gasification, bamboo-based charcoals with different potassium contents were prepared (Table 3). B-0.5 was as-carbonized. To reduce the potassium content, B-0.5 was thoroughly rinsed in water. On the other hand, to increase the potassium content, B-0.5 was dipped in an aqueous solution of potassium hydroxide of 5 % for 3 h under vacuum at room temperature. Then B-0.3 and B-0.9 were prepared. The activation behaviors for these samples were analyzed by measuring the TG curves (Figure 5). It is seen that a gasification rate was greatly accelerated with increasing the potassium content. In the case of B-0.9 enriched with potassium compounds, the weight monotonously decreased with increasing time, which means that the apparent activation reaction can be assumed as zero order. The gasification reaction seems to proceed evenly in every tissue. As for B-0.5 or B-0.3, the gasification rate suddenly increased at a certain treatment time. This is probably due to the deviation in the distribution of potassium compounds. The potassium content in secondary wall is lower than other portions. When the gasification reaction propagates into the intercellular regions, the potassium will work as a catalyst and accelerate the reaction (Figure 3).
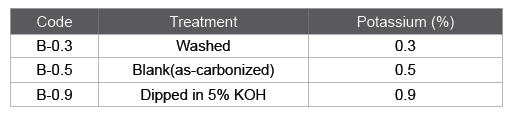
Table 2: Heterogeneous contaminative components in charcoal produced at 800 centigrade, %.
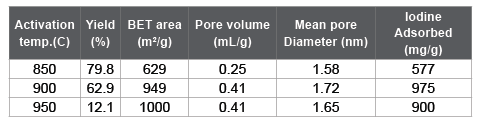
Table 3: Preparation conditions for bamboo-based charcoals with different potassium contents.
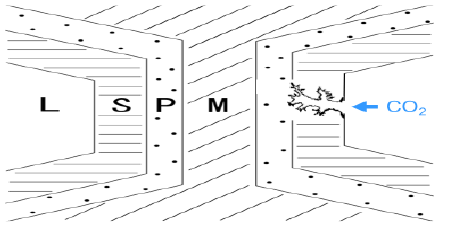
Figure 3: Schematic representation for the activation process in the tissue of bamboo: L, lumen; S, secondary wall; P, primary wall; M, middle lamella
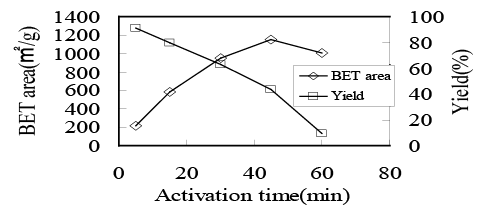
Figure 4: Relations between activation time and BET surface area or yield of AC produced at 900 centigrade in 50% CO2 .
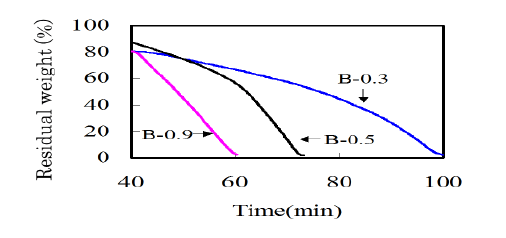
Figure 5: TG curves of bamboo-based charcoals with various potassium contents measured at 900 centigrade in 50% CO2.
The pore size distribution is one of the most important properties for ACs and is influenced by the activation conditions. Figure 6 shows cumulative pore volume curves for the bamboo-based ACs with different potassium content. The AC obtained from B-0.3 showed a steeper increase, which suggests that a sharper pore size distribution around 0.5 nm was existing in the AC from B-0.3. In order to produce the ACs from bamboo with a sharp pore size distribution, it is important to control alkali metal contents.
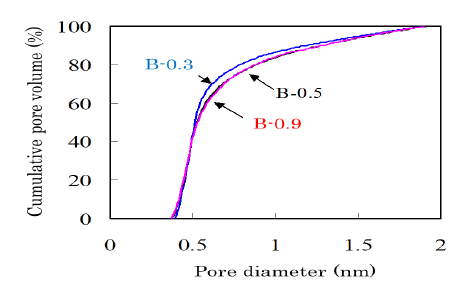
Figure 6: Cumulative pore volume curves in the range of diameter smaller than 2 nm measured for the ACs produced from bamboobased charcoals with various potassium contents. The yield of AC was controlled at 70%.
The adsorption tests were made for the ACs summarized in table 4. AC-C and AC-B were the commercial ACs from coconut shell and bituminous coal, respectively. BW-70, B-70, and B-40 were produced from bamboo. R-30 was the AC produced from the reed grass-based charcoal. Figure 7 shows removal percentages against 2-MIB and geosmin. The ACs produced from bamboo or reed grass showed a comparable or a little superior removal percentage as compared with commercial ones although the BET surface areas of BW-70, B-70 were much smaller. It seems that bamboo is a suitable starting material to produce AC for the removal of musty and earthy off-flavors. Reed grass was rich in the siliceous compounds. R-30 prepared by the activation of the reed grass-based charcoal showed surface values similar to commercial AC-B although the removal percentage of smells was much greater. At present it is difficult to discuss deeper from the limited surface data. However, it seems that the siliceous compounds in reed grass hardly affected its activation reaction.
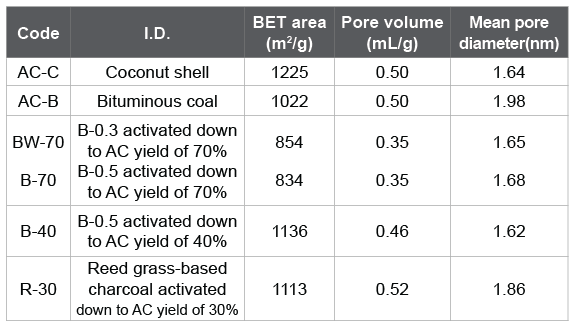
Table 4: Surface properties for ACs.
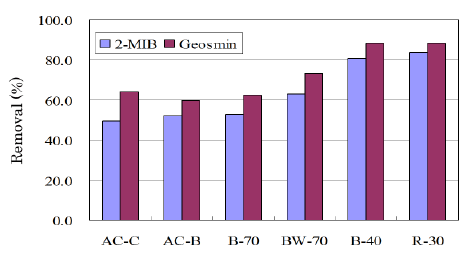
Figure 7: Removal percentages for ACs against 2-MIB, geosmin.
Similar tests were made against VOCs (Figure 8). There observed little difference between the ACs from bamboo-based charcoals and commercial ones except for the data against ammonia.
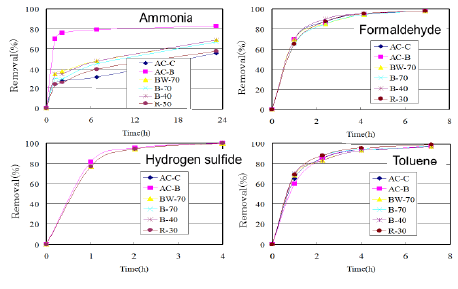
Figure 8: Removal percentages for ACs against VOCs.
The investigations were made on the carbonization and the activation behaviors of bamboo, and the applicability of bamboo to the production of high functional AC for the removal of musty and earthy off-odors and VOCs was discussed. In the activation process, it was found that the potassium fractions mainly settled in the intercellular regions worked as a catalyst and accelerated the activation reaction. Therefore, when the activation reaction propagated into the intercellular regions, the activation reaction was suddenly accelerated, leading to the wider and featureless pore size distribution of resultant AC. In order to obtain AC with sharper pore size distribution from bamboo, it is important to reduce the amount of potassium compounds.
The absorption tests were made using the ACs from bamboo-based charcoals, and it was confirmed that those ACs could have a little superior absorption properties against musty/earthy off-odors in the liquid-phase adsorption and almost comparable against formaldehyde, hydrogen sulfide, and toluene in the gas-phase adsorption as compared with commercial ACs.
Download Provisional PDF Here
Article Type: Research Article
Citation: Kawahara Y, Wakizaka H, Yamamoto K, Ishibashi N (2016) Preparation of BambooBased Carbonaceous Adsorbents for the Removal of Musty/Earthy Off-Odors and Vocs. Int J Water Wastewater Treat 2(4): doi http://dx.doi. org/10.16966/2381-5299.124
Copyright: © 2016 Kawahara Y, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Publication history:
All Sci Forschen Journals are Open Access