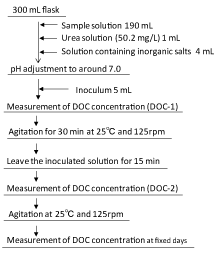
Figure 1: Procedure for biodegradation test

Nobuyuki Takahashi1* Hiroyasu Ichikawa1 Fumio Kiyono1 Toshiyuki Hibino1 Hisatomo Torii2 Shingo Shibata2 Tuyen Trinh Van3
1Institute for Environmental Management Technology, National Institute of Advanced Industrial Science and Technology (AIST), Japan*Corresponding author: Nobuyuki Takahashi, Institute for Environmental Management Technology, National Institute of Advanced Industrial Science and Technology (AIST)16-1 Onogawa, Tsukuba, Ibaraki 305-8569, Japan, Tel: +81-29-861-8727; Fax: +81-29-861-8722; E-mail: takahashi-nobuyuki@aist.go.jp
The degradation of 1,4-dioxane was investigated on a laboratory scale to estimate the enhancement of biodegradability and the effect of the reaction products on the water environment. The degradation of 1,4-dioxane by ozonation with hydrogen peroxide was proceeded rapidly compared with that by ozonation at pH 6 to 8. As a result, the biodegradability represented as the ratio of biochemical oxygen demand to dissolved organic carbon of the reaction products was higher than that of original 1,4-dioxane. The decrease in dissolved organic carbon concentration of the reaction products was observed during the incubation in the biodegradation test, showing the enhancement of biodegradability. The biotoxicity of the reaction products measured by the Microtox test showed an initial increase followed by a decrease with increasing reaction time.
1,4-Dioxane; Advanced oxidation process; Biodegradability; Biodegradation test; Microtox test
1,4-Dioxane is widely used as a solvent in the manufacture of organic chemicals; a stabilizer for chlorinated solvents, such as 1,1,1-trichloroethane, and a wetting agent in paper and textile processing [1]. 1,4-Dioxane is also the by-product of several chemical processes involving ethylene glycol or ethylene oxide [2]. 1,4-Dioxane is classified as Group 2B by the International Agency for Research on Cancer (IARC) because it is possibly carcinogenic to human [1]. In Japan, the new environmental standard of 0.05 mg/L was adopted in 2011 and the drainage regulation standard of 0.5 mg/L was also adopted in 2012. In this regard, the development of a new cost-effective treatment system is strongly desired.
Many studies of the degradation and/or removal of 1,4-dioxane have been conducted. Those studies have shown that 1,4-dioxane is not effectively treated by conventional methods, including biological treatment, coagulation, and activated carbon adsorption, and that advanced oxidation process (AOP) using hydroxyl radical (HO• ) shows the effective degradation of 1,4-dioxane. As AOPs, ozonation with UV irradiation (O3 UV) [3,4], ozonation with hydrogen peroxide (O3 /H2 O2 ) at pH 6 to 8 [4-6], ozonation with electrolysis [7], UV irradiation with hydrogen peroxide [8], and UV irradiation with titanium oxide [9,10] have been mainly reported. Those studies have also shown the superiority of AOPs to ozonation alone. Among those AOPs, O3 /UV and O3 /H2 O2 have been frequently reported. However, the treatability with O3 /UV is expected to decrease because the permeability of UV irradiation becomes ineffective in colored wastewater. This suggests the higher applicability of O3 /H2 O2 than O3 /UV in real wastewater treatment.
Adamus et al. [5] and Suh et al. [6] have indicated that the degradation of 1,4-dioxane by O3 /H2 O2 enhanced biodegradability in the treated water. Adamus et al. [5] and Takahashi et al. [4] have also showed that there is a desirable molar ratio of H2 O2 to O3 . However, the effect of the reaction products of 1,4-dioxane by O3 /H2 O2 on the water environment has been hardly investigated, and detailed studies of whether or not the organic compounds derived from the degradation of 1,4-dioxane are further decreased on the water environment are necessary.
In this study, the degradation of 1,4-dioxane by O3 /H2 O2 as a practical AOP was conducted on a laboratory scale. Afterward, the biodegradability of the reaction products derived from 1,4-dioxane was estimated, and the effect of the reaction products on the water environment was investigated by the biodegradation test using surface water and the Microtox test.
1,4-Dioxane was purchased from Wako Pure Chemical Industries, Japan. Taking the average concentrations of 1,4-dioxane in real wastewater at a chemical manufacturing factory into account, 150 mg/L solutions of 1,4-dioxane was prepared using distilled and deionized water.
O3 /H2 O2 at pH 6 to 8 was conducted using the semibatch-type ozonation apparatus [4]. 30% H2 O2 solution (Wako Pure Chemical Industries, Japan) of 0.1, 0.3, and 0.6 mL was added to 1.8 L of 1,4-dioxane solution before each experiment, because an excess amount of H2 O2 influenced on biochemical oxygen demand (BOD5 ) measurement [4]. The concentrations of H2 O2 were approximately 16.7, 50.1, and 100.2 mg/L, respectively. A 1.6 L sample solution was placed in a reactor of 30 cm height and 9.5 cm inner diameter and treated by bubbling ozonized gas at a flow rate of 0.5 L/min. Ozonized gas containing 34.1~36.2 mg-ozone/L was generated with an ozone generator (ON-1-2, Nippon Ozone, Japan). Ozonation was conducted for 4 h at ambient temperature. As the reference treatments, ozonation at pH 6 to 8 without H2 O2 solution, aeration of oxygen gas, and addition of H2 O2 solution were also conducted. The pH of the solutions was controlled from 6 to 8 by adding 1 N HCl or 1 N NaOH.
Ozone concentrations in the inlet gas and the exhaust gas were measured by the KI method, respectively, and the amount of ozone consumed in the reactor was determined from the difference of them. In order to evaluate the further degradation of intermediate products derived from the degradation of 1,4-dioxane, dissolved organic carbon (DOC) and BOD5 were measured. DOC was measured with a TOC analyzer (TOC-500, Shimadzu, Japan). BOD5 was measured according to JIS K0102. 1,4-Dioxane was measured with a high-performance liquid chromatography (HPLC) (LC-10, Shimadzu, Japan) equipped with a RI detector (RID-10, Shimadzu, Japan). The analytical conditions were as follows; mobile phase: water, flow rate: 1 mL/min, oven temperature: 40°C, column: COSMOSIL 5C18-PAQ (Nacalai Tesque, Japan) [7]. The effect of 1,4-dioxane and its reaction products on the water environment was investigated by both the 10-day biodegradation test and the Microtox test. In the Microtox test, bio-toxicity (TU=100/EC50) was measured with a thermostatic photometer (Model 500, Microbics Inc., USA) and freezedried P. phosphoreum (Modern Water Inc., USA). Figure 1 shows the procedure for the 10-day biodegradation [11]. Surface water from Ohori River as inoculums, the volume of sample solution was 190 mL and no deionized distilled water was added for dilution.
As the reference treatments, the degradation and/or removal of 1,4-dioxane by ozonation at pH 6 to 8, aeration of oxygen gas, and addition of H2 O2 solution were first conducted to compare with the treatability of O3 /H2 O2 . Figure 2 shows the change of water quality parameters during the degradation of 1,4-dioxane by O3 at pH 6 to 8. Slight degradation of 1,4-dioxane was observed, and the decrease in DOC was only 7~8 mg/L. The degradation of 1,4-dioxane by ozonation at pH 6 to 8 was expected to proceed with the production of HO • [12] although the extent of degradation was slight. The removal of 1,4-dioxane by aeration was reported to increase with increasing aeration intensity [13]. However, no decrease was observed in this study because of the low aeration intensity of 30 L-ozonized oxygen gas/h to 1.6 L solution. Moreover, 1,4-dioxane was not degraded by addition of H2 O2 solution. These results indicate that the extents of degradation and/or removal of 1,4-dioxane by ozonation at pH 6 to 8, aeration, and addition of H2 O2 solution were very limited.

Figure 1: Procedure for biodegradation test
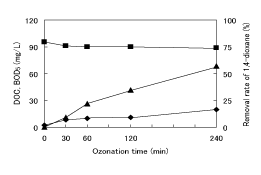
Figure 2: Change of removal rate of 1,4-dioxane (▲) and the change of DOC (■) and BOD5 (♦) by O3 at pH 6 to 8
Figures 3A and 3B shows the change of water quality parameters during the degradation of 1,4-dioxane by O3 /H2 O2 . The degradation of 1,4-dioxane by O3 /H2 O2 was accelerated compared with that by ozonation at pH 6 to 8. 1,4-Dioxane at the initial concentration of 150 mg/L was completely degraded by 1~2 h reaction. During the degradation, both the decrease in DOC and the appearance of a maximum value for BOD5 were observed. The comparison between Figures 3A and 3B showed that the oxidation potential affected the residual concentration of DOC and/or BOD5 as well as the degradation of 1,4-dioxane. As shown in Figure 3A, large amount of DOC and/or BOD5 remained in the solution after O3 / H2 O2 with low oxidation potential. On the other hand, as shown in Figure 3B, the increase in the amount of H2 O2 added from 0.1 to 0.6 mL caused both the further decrease in the residual concentration of DOC and/or BOD5 . These results show the effective degradation of 1,4-dioxane by O3 / H2 O2 compared with ozonation at pH 6 to 8.
In order to estimate the change of biodegradability during the degradation of 1,4-dioxane, the ratio of BOD5 to DOC (BOD5 /DOC) were calculated using BOD5 and DOC obtained from the degradation of 1,4-dioxane [11]. Figure 4 shows the change of BOD5 /DOC with and without H2 O2 addition. As shown in Figure 4, BOD5 /DOC showed a steady increase and/or a maximum, because DOC steadily decreased and BOD5 showed a maximum with increasing reaction time. The increase in BOD5 /DOC indicates the accumulation of biodegradable intermediates, and the maximum suggests the further degradation of the intermediates with increasing reaction time.
AOP, using HO+ as main reaction species, shows the effective degradation of 1,4-dioxane. Several researchers have investigated the reaction pathway of 1,4-dioxane [14,7-10]. Table 1 summarizes the reaction products derived from the degradation of 1,4-dioxane. Figure 5 shows the initial degradation mechanism for 1,4-dioxane proposed by Beckett et al. [14]. 1,4-Dioxane was first degraded to initial intermediates, such as ethylene glycol diformate and ethylene glycol monoformate, by HO+. These initial intermediates are further degraded to some low molecular weight organic compounds, and then, those compounds were finally degraded to CO2 and H2 O. As a reaction pathway after the production of ethlene glycol diformate, Figure 6 shows the degradation pathway of 1,4-dioxane proposed by Maurio et al. [7]. The production of CO2 indicates the decrease of DOC concentration, as shown in Figure 3. In addition, the BOD5 /DOC values of some low molecular weight organic compounds of glycolaldehyde, formic acid and oxalic acid in Figure 6 are 1.06, 0.50 and 0.32, respectively [15], and those are higher than that of 1,4-dioxane of less than 0.05. The result indicates the enhancement of biodegradability induced by O3 /H2 O2 , leading to a decrease in the load on the water environment accepting the discharged effluents.
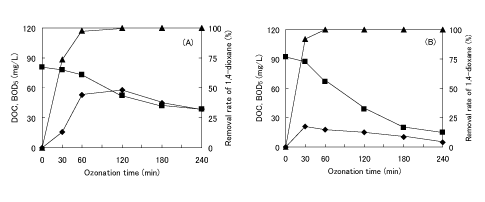
Figure 3: Change of removal rate of 1,4-dioxane (▲) and the change of DOC (■) and BOD5 (♦) by O3 /H2 O2 at the amount of H2 O2 added of 0.1 mL (A) and 0.6 mL (B).
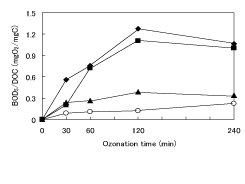
Figure 4: Change of BOD5 /DOC during the degradation of 1,4-dioxane by O3 /H2 O2 . Amount of H2 O2 added (mL); ○:0, ■:0.1, ♦:0.3, ▲:0.6.
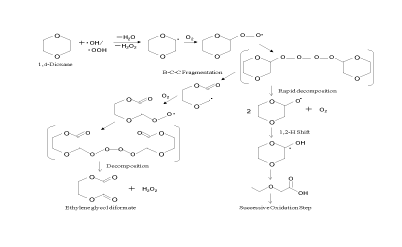
Figure 5: Reaction scheme of the initial degradation mechanism for 1,4-dioxane by Beckett et al. (2000).
In order to clarify the enhancement of the biodegradability suggested in the former section, 1,4-dioxane was treated with O3 /H2 O2 and then, the biodegradation test using surface water was conducted on both the original 1,4-dioxane and the treated water. Figure 7 shows the change of the concentration of DOC during the biodegradation test. Figure 8 shows the decrease in DOC concentration (ΔDOC) calculated from Figure 7. 0 on the left of the plots in Figure 7 corresponds to DOC-1 in Figure 1, and 0 on the right to DOC-2 in Figure 1, respectively. As shown in Figure 7, DOC concentration in the treated water was decreased with incubation days, and this tendency was not found in the original 1,4-dioxane solution. Moreover, the extent of the decrease was increased with increasing ozonation time, as shown in Figure 8. These results actually show the enhancement of biodegradability suggested in Figure 4, and indicate the effectiveness of O3 /H2 O2 .
On the other hand, the initial peak areas of 1,4-dioxane in HPLC at 0, 30, 60 120 min were 16569, 5601, 827 and 0, respectively, and those were not changed during incubation, indicating that the concentration of 1,4-dioxane was almost constant in both the original 1,4-dioxane and the treated water, regardless of ozonation time. Therefore, the degradation of 1,4-dioxane is generally impossible on the water environment, and that the discharge less than the criteria is necessary.
As shown in Figure 7, DOC components will probably remain in the treated water in proportion to ozonation time and incubation day. In order to investigate the bio-safety of the treated water, the Mocrotox test was conducted. The Microtox test measures the extent of light emission in a sample solution using photobacterium, and can yield results within short-term reaction [16]. Taking this characteristic into account, the bio-toxicities after 5- and 15-min reactions (TU (5) = 100/EC50 and TU (15)=100/EC50) were measured. Figure 9 shows the change of bio-toxicity after 5- and 15-min reactions. Bio-toxicity showed a maximum at 30~60- min reaction, and decreased thereafter to a value lower than the detection limit. These results show that in the case of short-term reaction, bio-toxic intermediates will remain in the solution and the intermediates will be further degraded to bio-safe compounds with reaction time. As a result, the establishment of desirable reaction conditions under which bio-toxic intermediates do not remained in the solution is very important.
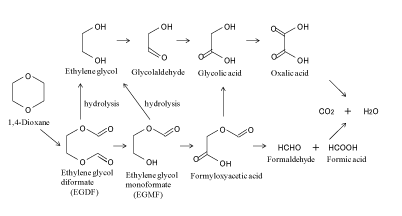
Figure 6: Proposed reaction pathway of 1,4-dioxane degradation by Maurio et al. (1997).
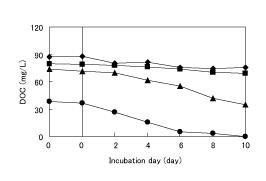
Figure 7: Change of concentration of DOC during biodegradation test at the amount of H2 O2 added of 0.3 mL. Ozonation time (min);♦:0, ■:60, ▲:120, ●:240.

Figure 8: Decrease in DOC concentration during biodegradation test at the amount of H2 O2 added of 0.3 mL. Ozonation time (min);♦: 0, ■:60, ▲: 120, ●:240.
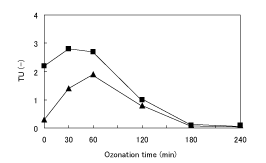
Figure 9: Change of TU after 5-min (▲) and 15-min (■) reaction at the amount of H2 O2 added of 0.6 mL.
The degradation of 1,4-dioxane by O3 /H2 O2 was investigated on a laboratory scale. The biodegradability of the reaction products was estimated, and the effect of the reaction products on the water environment was measured by the biodegradation test using surface water and the Microtox test. The following conclusions were obtained:
(1) The degradation of 1,4-dioxane by O3 /H2 O2 was proceeded rapidly compared with that by ozonation at pH 6 to 8.
(2) The biodegradability represented as the ratio of BOD5 to DOC of the reaction products was higher than that of original 1,4-dioxane.
(3) The decrease in DOC concentration of the reaction products was observed during the incubation, showing the enhancement of biodegradability.
(4) The bio-toxicity of the reaction products measured by the Microtox test showed an initial increase followed by a decrease with increasing reaction time.
The authors are very thankful for the financial support from the New Energy and Industrial Technology Development Organization (NEDO).
Download Provisional PDF Here
Aritcle Type: Research Article
Citation: Takahashi N, Ichikawa H, Kiyono F, Hibino T, Torii H, et al. (2015) Enhancement of Biodegradability of 1,4-Dioxane induced by O3 /H2 O2 . Int J Water and Waste Water Treatment 1(1): doi http://dx.doi.org/10.16966/2381-5299.105
Copyright: © 2015 Takahashi N, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Publication history:
All Sci Forschen Journals are Open Access