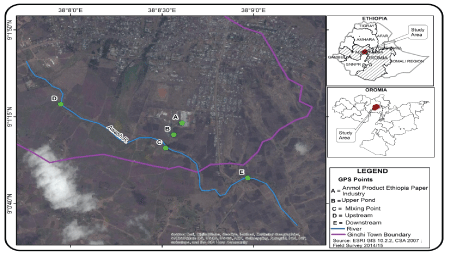
Figure 1: Location maps of the study area and sampling site.


Zerihun Feyissa Jebessa1 Eshetu Bekele Wondemagegnehu2*
1Department of Chemistry, Bule Hora University, Ethiopia*Corresponding author: Wondemagegnehu EB, Department of Applied Chemistry, Adama Science and Technology University, Ethiopia, Tel: +251911628565; E-mail: eshetubekele@gmail.com
Pulp and paper factory effluents entering in to water bodies have been considered as major sources of pollutants in the environment. The extent of pollution depending on the nature of raw materials and the technology used in all stages of paper making process. In this study, the physicochemical characteristics of effluents from Anmol Product Ethiopia Factory (APEPF) and the receiving upper Awash River water were investigated. Composite samples, with a total sample volume of 2 L from each site was collected for 12 hours and in the month of January to March, 2015 of the Ethiopian dry season using polyethylene sample bottles. Sample preservation, storage, pretreatments and psychochemical characterization were performed according to standard procedures recommended by American Public Health Association (APHA, 2005). Mean comparison were made to examine spatial variations of physicochemical characteristics along the river water as a result of discharged effluents from the factory. Moreover, the results were compared with the National (EEPA, 2010) and WHO (2008) standards. The results showed that the raw effluent comprised of 298-501 mg/L of Total Suspended Solids (TSS), 1032-2333 mg/L of Total Dissolved Solids (TDS), and pH varied from 3.2-10.7. The five day Biological Oxygen Demand (BOD5) and Chemical Oxygen Demand (COD) of the raw effluent ranged from 470-2499 mg/L and 2969-5847 mg/L, respectively. Aerobic treatment of raw effluent were not significantly reduced TSS (112-532 mg/L), BOD5 (405-1315 mg/L), and COD (2304-3729 mg/L). Moreover, TDS, Electrical Conductivity (EC), Total Nitrogen (TN), Total Phosphorus (TP), Potassium (K), Chlorides (Cl-), and Sulphates (SO42-) were found superior after treatment. The values of most of these parameters were found higher than the Ethiopian Environmental Protection Authority (EEPA) and World Health Organization (WHO) limit prescribed for industrial effluent discharge. The ANOVA result indicated the levels of measured parameters were highest at the point of entry followed by downstream samples, and the least value was recorded at the upstream samples. Moreover, the recorded values were much higher than the maximum allowable limit even at downstream of the river. The dendrogram of hierarchical clustering clearly indicated that effluents from APEPF contributed to the change in composition of river water samples taken from downstream of the river. All these confirmed APEPF effluent discharged to the river was not in accordance with both national and WHO standards and found to have considerable effect on the upper Awash River water quality.
Awash river; Physicochemical characteristics; Pulp and paper; Industry effluents
The pulp and paper manufacturing sector is considered as one of the major potential sources of pollution in the environment [1,2]. In general, process of paper making involves the use of large amounts of water, chemicals, additives and fillers, consequently discharging large volume of black liquor containing lignin and hemicellulose, toxic waste materials like dimethylsulphite into the environment along with high COD [3-6]. Moreover, the pollutants released from paper factory are rich in dissolved solids such as chlorides and sulphates of Na, Ca, and suspended organic materials [3,7]. The direct discharge of such effluents into aquatic systems spoils fresh water ecosystems that resulted in series of problems for both aquatic life and different uses of water [7-9]. The high organic content of pulping effluent coupled with chlorine from the bleaching process results in the production of highly toxic organic compounds such as chlorinated phenols, furans and aliphatic hydrocarbons [10]. These compounds are known to be toxic, mutagenic, persistent, and bio-accumulating that causes harmful disturbances in biological systems, and human health risk [2,11]. This urges the need to control the quality of the effluents.
In recent years, related to increasing industrialization and fast developmental activities in Ethiopia, the existing and newly established pulp and paper industries are expected to fulfill the increasing demand for paper. These industries mainly use imported pulp and used paper for paper production, and they were established along the Awash River. The local communities near and downstream of this river have been heavily utilized it for different domestic and agricultural purposes [12]. However, reports confirmed that the river contained high concentration of salts, organic wastes and pathogenic organisms because of a number of anthropogenic activities that release their wastes into the river [13]. The local community recognized this effect as the colour of the river has changed and fish in the river have slowly disappeared. Therefore, for any sustainable interventions, assessing effluent physicochemical characteristics and their effects to the river water quality is needed.
A number of studies related to physicochemical characterization of the pulp and paper factory effluents were conducted in different countries to investigate the extent of pollution to the receiving water body [5,11,14,15]. The results confirmed that the effluents of the pulp and paper factory were loaded with many pollutants in which the volume and characteristics of the pollutants vary depending on the type of raw materials, manufacturing processes, amount of water and adopted treatment technologies used [16]. For instance, in effluents from the Lalkuan Paper Factory, India, BOD5 (306-408 mg/L), COD (1736-4357 mg/L), Cl- (43.5-348 mg/L), and TDS (486-1380 mg/L) were found [11]. Relatively higher amounts of BOD5 (870 mg/L), COD (2000mg/L), TS (2890 mg/L) were also determined in a pulp and paper factory effluent in Bhilai, India [17]. Jamil TS, et al. [18] also found extremely higher BOD5 (2200 mg/L), COD (10,300 mg/L), TS (5950 mg/L) in Egyptian recycled pulp and paper factory effluents. However, in Ethiopia, studies related to type, transport and effects of pollutants released from APEPF were not studied.
Therefore, the present study aimed to investigate the physicochemical characteristics of effluent discharged by APEPF and its subsequent effect on the water quality of Awash River.
The study area is found in Oromia region of Ethiopia and located between 38°8’0”E to 38°9’0”E and 9°0’40”N to 9°1’50”N. It has an average annual temperature of 23.8°C and receives a mean annual rainfall of 1172 mm. It is characterized by a bimodal rainfall pattern, during summer (July-September) and spring (April-May). It is situated at an altitude of 3000 m as l with dry mid land climate condition.
At the study area, APEPF was established in 2009, near the upper Awash River and it spreads over 13-hectares. The factory produces paper from used paper collected from different parts of the country as well as finished pulp imported from Indonesia. The factory uses a considerable amount of water from two sedimentation sources pumped to the production section through plastic pipes. Finally, the processed liquid effluent from the mill section was pumped to a storage pond located within the factory compound. The pulp collected from the storage pond is re-used in the mill, whereas the effluent leaving the pond is directly discharged to the upper Awash River. The Awash River basin is divided into upper, middle and lower valleys. The river originates from the central Ethiopian highlands at elevation 3000 m west of Addis Ababa and flows north east wards, crosses the Rift valley and finally enters into Lake Abe at an elevation of 250 m after covering a total distance of 1200 km. APEPF is discharging its effluent directly into the upper Awash River valleys.
Effluent samples of the factory and water samples from the upper Awash River were collected from five different sampling sites designated as sites A, B, C, D, and E (Figure 1). For the analysis of both metals and other physicochemical parameters, two sets of effluent and river water samples were collected from each sampling site with a total sample volume of 2 L each. Effluent samples were collected when it was pumped into the factory oxidation pond located near the mill (A), and after the effluent left the pond (B). Water samples of the upper Awash River were collected at the point of discharge (C), from unpolluted site located at about 1 km upstream of the effluent entry point (D), and at a distance of 1 km downstream of the effluent entry point (E). Sample bottles were previously washed carefully with detergent and 1M H2SO4 and rinsed by water samples at the point of collection. Composite samples of both the effluent and the river water samples were collected for 12 hours, well mixed and in the month of January to March, 2015 of the dry season. The samples were taken in every fifteen days interval using polyethylene sample bottles. Samples were transported to Jije Labo Glass Analytical Laboratory service, Addis Ababa within 2 hours of collection and refrigerated at 4°C. Prior to treatment, the samples were warmed to room temperature (21 to 25°C). Sample collection, preservation and storage were performed according to standard procedures recommended by American Public Health Association (APHA 2005) [19].

Figure 1: Location maps of the study area and sampling site.
All the reagents and chemicals used in this study were analytical grade. Hydroxylamine buffer solution, disodium hydrogen phosphate (pH=7.4) and borax solution (pH=9.2) (BDH Chemicals Ltd. England) were used for measurement of samples pH 0.25N potassium dichromate solution (Breckland Scientific LTD), 0.25N ferrous ammonium sulfate, Ferroin indicator (Eurostar Scientific LTD), and powder mercury sulfate were used for COD measurement. 8.0N sodium hydroxide, manganous sulphate, sodium iodide azide reagent, conc.H2SO4 , 0.025N Na2S2O3 solution and starch indicator were used for Biological Oxygen Demand (BOD) determination. Methyl red-Bromocresol mixed indicator, 40% NaOH, 2% boric acid, conc. HNO3 , vanadomolybdate, conc. HCl, H3PO4 , H3BO3 buffer solution for the determination of total phosphorus. Copper sulphate and Potassium sulphate mixture for the determination of total nitrogen were all supplied by CDH Chemicals Ltd., England. Calmagite indicator and 0.02N EDTA solution for total hardness determination, phenolphthalein indicator, methyl orange indicator and 0.02N H2SO4 solution used for the determination of total alkalinity, standard 0.014N silver nitrate solution and potassium chromate indicator used for the measurement of Chloride, Sulfa Ver 4 reagent. Na2SO4 standard solution, powder sodium ascorbate, Zincon reagent and NH2OH used for sulphate determination, HCl solution used for Zinc metal determination. Sodium metabisulfite solution, diphenylcarbazide reagent and Phenanthroline reagent used for total iron determination and neocuproine reagent used for copper metal determination were supplied by HACH Scientific LTD, USA. Distilled water was used for dilution of sample and metal standard solutions as well as for rinsing glassware and sample bottles.
The pH of APEPF effluents and upper Awash River water samples was determined at room temperature by Systronic digital pH meter (HI 9023 model). The standardization of the instrument was done with a buffer solution of disodium hydrogen phosphate (pH=4.7) and borax (pH=9.2). The electrical conductivity was determined by using a conductivity meter (model HI 8733). Sulphate and turbidity of each sample were measured turbidmetrically using UV-V is Spectrophotometer (HACH Scientific LTD 6305 model, USA). Na and K was determined photometrically using Flame photometer (Halseled-410 model). Copper, Zinc and Iron concentrations in all samples were measured colorimetrically using UV-Vis Spectrophotometer (HACH Scientific LTD 6305 model, USA). Total Phosphorous was measured by colorimetric method using Vanadomolybdophosphoric acid reagent by UV-Visible Spectrophotometer (T/70/T80 model, China). Alkalinity, total Hardness, chloride and total Nitrogen was analyzed titrimetrically (Kjeldahl method) and Suspended Solid (SS) and Total Dissolved Solid (TDS) were measured gravimetrically. BOD5 was measured from the difference of dissolved oxygen in the samples measured by Winkler’s iodometric method before and after incubation at 20°C in BOD incubator (SPX 250B model, china) for five days. COD was analyzed using an open reflux titrimetric method. All the physicochemical parameters were analyzed using the standard methods of American Public Health Association (APHA 2005) [19].
A blank was prepared using distilled water and reagents for the characterization of each physicochemical parameter. Samples were analyzed in triplicate and instrument calibration carried out with a range of standard solutions. For UV-Vis spectrophotometry and flame photometer determination five working calibration standards were prepared by serial dilution of concentrated stock solution of each metal. A calibration curve of absorbance versus concentration was established for each metal and used for determination of concentration in the samples of effluent and river water.
The raw data of both effluent and river water physicochemical parameters were checked for normal distribution and analyzed using SPSS version 15.0 software. This was followed by the Least Square Difference (Fischer LSD) test to compare means results obtained from the physicochemical analysis of effluents and among water samples collected at the different sampling points of the river. The coefficient of correlation between some physicochemical parameters was calculated by Pearson correlation test at 0.05 and 0.01 significant levels. Hierarchical Cluster Analysis (HCA) was used to classify both effluent and river water samples into different groups based on similarities in measured water quality parameters using R-software.
Mean values of the physicochemical characteristics of both raw and treated effluents in comparison with the National and WHO standards were shown in table 1.
| Parameter | Condition | Min. | Max. | Mean ± SD | Discharge permissible limit | |
| WHOa | EEPAb | |||||
| BOD5 (mg/L) | Raw | 470 | 2499.3 | 1176.84 ± 88.10 | 50 | 40 |
| Treated | 405 | 1314.6 | 886.7 ± 63.01 | |||
| COD (mg/L) | Raw | 2969 | 5848.6 | 4065.86 ± 301.30 | 250 | 120 |
| Treated | 2304 | 3729.6 | 2947.2 ± 224.19 | |||
| TP(mg/L) | Raw | 0.37 | 0.42 | 0.39 | 2 | - |
| Treated | 0.35 | 1.69 | 1.02 ± 0.03 | |||
| TN (mg/L) | Raw | 7.79 | 20 | 13.89 ± 0.74 | 10 | - |
| Treated | 12.41 | 12.80 | 12.6 ± 0.25 | |||
| Turbidity (NTU) | Raw | 118.28 | 499.32 | 270.35 ± 11.09 | 5 | 5 |
| Treated | 50.88 | 418.65 | 198.12 ± 7.24 | |||
| TS (mg/L) | Raw | 1512 | 3432 | 2592.67 ± 297.99 | 1000 | |
| Treated | 1230 | 2854 | 2197.33 ± 210.94 | |||
| TDS (mg/L) | Raw | 1032 | 3134 | 2333.33 ± 312.67 | 1000 | 1000 |
| Treated | 1118 | 2650 | 2092 ± 268.`9 | |||
| TSS (mg/L) | Raw | 298 | 726 | 501.33 ± 62.67 | 200 | 100 |
| Treated | 112 | 532 | 282.66 ± 28.44 | |||
| EC (µS/cm) | Raw | 1576 | 4720 | 3198.67 ± 0.24 | 1000 | 1000 |
| Treated | 1705 | 4020 | 2888.33 ± 0.36 | |||
| PH | Raw | 3.23 | 10.66 | 6.83 ± 0.10 | 5-9 | 5.5-9 |
| Treated | 3.4 | 10.3 | 6.47 ± 0.12 | |||
| Na (mg/L) | Treated | 140 | 900 | 556.67 ± 16.59 | 400 | 400 |
| Raw | 130 | 800 | 493.33 ± 12.83 | |||
| K (mg/L) | Treated | 2.9 | 12.1 | 6.57 ± 0.18 | - | - |
| Raw | 2.1 | 11.6 | 8.23 ±0.21 | |||
| Total Hardness (mg/L) | Raw | 49.5 | 3335 | 1394.83 ± 58.5 | - | - |
| Treated | 47.52 | 3036 | 1291.17 ± 65.84 | |||
| Ca (mg/L) | Raw | 11.09 | 1150 | 483.03 ± 22.79 | 200 | 200 |
| Treated | 8.71 | 1104 | 464.23 ± 16.63 | |||
| Mg (mg/L) | Raw | 5.23 | 110.4 | 44.94 ± 1.38 | 150 | 150 |
| Treated | 6.18 | 66.24 | 31.34 ± 0.95 | |||
| Alkalinity (mg/L) | Raw | ND | 2000 | 1032.68 ± 64.62 | - | - |
| Treated | ND | 1700 | 953.09 ± 57.18 | |||
|
CO32- (mg/L) |
Raw | ND | 281 | 93.89 ± 5.08 | - | - |
| Treated | ND | 389.42 | 129.81 ± 6.25 | |||
|
HCO3- (mg/L) |
Raw | ND | 2440 | 1068.97 ± 71.04 | - | - |
| Treated | ND | 2074 | 898.84 ± 58.33 | |||
| Chloride (mg/L) | Raw | 186.22 | 3818.7 | 1454.98 ± 101.13 | 1000 | 750 |
| Treated | 150.41 | 815.44 | 419.33 ± 25.22 | |||
| Sulphate (mg/L) | Raw | 26.7 | 282.32 | 195.31 ± 12.76 | - | - |
| Treated | 219.25 | 424.22 | 309.09 ± 14.85 | |||
| Fe (mg/L) | Raw | 0.27 | 2.77 | 1.42 ± 0.02 | ||
| Treated | 0.18 | 1.67 | 1.00 ± 0.01 | |||
| Cu (mg/L) | Raw | ND | 0.03 | 0.01 | 2 | 2 |
| Treated | ND | 0.03 | 0.01 | |||
| Zn (mg/l) | Raw | ND | 0.59 | 0.2 | 10 | 6 |
| Treated | 0.13 | 0.55 | 0.34 | |||
Table 1: Mean values (n=3) of physiochemical characteristics of Anmol product Ethiopia paper factory effluents collected for two consecutive months (January to March, 2015) with standard discharge limits.
aWHO (2008) World Health Organization standard limit
bEEPA (2010) Ethiopian Environmental Protection Authority
The pH values ranged between 3.2 to 10.7 for the raw effluents, and 3.4 to 10.3 for the treated effluents (Table 1). Acidic pH values of the effluents taken during the first sampling data may be attributed to acidic digestion with salts of sulphites (SO32-) during pulp production. The basic pH values obtained for samples taken after two weeks confirmed that the factory uses NaOH liquor during digestion of used paper [16]. The value obtained for alkaline pulping process is comparable with the value reported by Nandkumar P, et al. [17] 11.5 for Kraft paper mill at M. Bhilai (India). The values obtained were not fulfilled either the national or the WHO permissible limits (pH of 5.0- 9.0) for the discharge of effluents into surface water.
As shown in table 1, EC levels of the factory effluents were considerably high, 1476-4720 µS/cm for the raw effluents and 664- 4020 µS/cm for the treated effluents. This indicated the presence of higher concentration of dissolved ions [15]. The mean conductivity values for both sampling points were higher than the National and WHO standards values of 1000 µS/cm for the discharge of effluents into the river water.
The level of TSS ranged from 298 to 726 mg/L for the raw effluents and 112 to 532 mg/L for treated effluents. These values were higher than the WHO permissible upper limit of 200 mg/L for the discharge of effluents into surface water (Table 1). These high values of TSS in the factory effluents may be due to the presence of non-dissolved substances including lignin and hemicelluloses generated from the raw material preparation, pulping, rinsing, bleaching and papermaking processes [9].
The TDS values ranged from 1032 to 3134 mg/L for the raw effluents and 1118 to 2650 mg/L for treated effluents. Similarly, the values of Total Solids (TS) ranged from 1512 to 3432 mg/L for the raw effluents and 1230 to 2854 mg/L for treated effluents (Table 1). These values of TDS and TS were higher than the national and WHO standards of 1000 mg/L for the discharge of effluents into surface water (Table 1).
Turbidity values for the raw and treated effluents were ranged from 118 to 499 NTU and 51 to 419 NTU, respectively (Table 1). The values obtained for turbidity were higher than the National and WHO effluents discharge limit of 5 NTU. This high turbidity of the effluents indicated the presence of various non-dissolved solid by-products during paper production process such as lignin and hemicelluloses [15].
The effluents had COD level of 2969 to 5849 mg/L for the raw effluents and 2304 to 3730 mg/L for the treated effluents (Table 1). The BOD5 level of the effluents obtained for the raw and treated effluents ranged between 470 to 2499 mg/L and 405 to 1315 mg/L, respectively. The level of` COD and BOD5 at both sampling points were higher than the WHO limit of 250 and 50 mg/L , respectively for the discharge of effluents into surface waters (Table 1). The BOD5 values of the effluents were higher than values reported by Giri J, et al. [11] and Kuzhali SS, et al [15], ranging from 306 to 408 mg/L from the pulp and paper mill effluent in India. However, it is comparable with the BOD5 value of 2200 mg/L in effluents released from the digester of used paper mill in Egypt [18]. The COD value was comparable with the value of 1736 to 4357 mg/L reported by Giriet J, et al. [2] for the pulp and paper mill effluents from the factory in India. The high BOD5 and COD values of APEPF effluents indicated the presence of oxygen demanding organic pollutants at higher level and inorganic pollutants, such as sulphides in which their oxidation also demand oxygen [7].
The BOD5/COD ratio is of great importance for quantification of biodegradability of any polluted effluent. A high ratio (>0.5) indicates good biodegradability and a ratio of <0.5 corresponding to low biodegradability of the organic material present in the effluents [18]. The mean BOD5 /COD ratio of 0.29 for the raw and 0.30 for treated effluents of APEPF indicated the effluents were dominated by nonbiodegradable organic molecules.
Total Nitrogen (TN) and Total Phosphorus (TP) concentrations of APEPF raw effluents ranged from 7.8 to 20.0 mg/L and 0.3 to 0.4 mg/L, respectively. Similarly TN and TP for treated effluent ranged from 12.4 to 12.8 mg/L and 0.4 to 1.7 mg/L, respectively (Table 1). The values of TN were higher than the WHO permissible limit of 10 mg/L and whereas the values of TP were below the permissible limit of 2 mg/L. The discharge of such effluents with high nutrients may cause eutrophication, consequently leading to the death of aquatic life because of oxygen depletion in the receiving water bodies.
The sulfate (SO4-2) concentration of the raw and treated effluents ranged from 26 to 282 mg/L and 219 to 424 mg/L, respectively (Table 1). The higher concentration of SO4-2 observed in the treated effluent, is attributed to sulphite oxidation to sulfate in well aerated zones [20]. The discharge of effluents with high sulphite content into a river stream may cause unpleasant odor and toxic to aquatic organisms (EEPA and UNIDO, 2003) [6]. On the other hand, as shown in Table 1, the concentration of chloride (Cl-) for the raw and the treated effluents during the study period ranged from 186 to 3819 mg/L and 150 to 815 mg/L, respectively. The Cl- content of effluents were significantly reduced downstream of the effluent from 3819 mg/L to 815 mg/L mainly as a result of reaction of chlorine with high organic content of the pulping effluents to form highly toxic organic compounds such as chlorinated phenols, furans, and aliphatic hydrocarbons [11,13]. However, the chloride contents for the raw and treated effluents were higher than the WHO permissible limit of 600 mg/L (Table 1). The high Cl- concentration indicated that chlorine containing agents were used by the factory in the pulp bleaching stage. The total hardness of the raw and treated effluents ranged from 50 to 3335 mg/L and 48 to 3036 mg/L, respectively (Table 1). The maximum values were categorized as hard effluents as it is above 150 mg/L [4].
The concentration of metals in the raw effluents ranged from Na (140-900 mg/L), K (2.9-12.1 mg/L), Ca (11.1-1150 mg/L), and Mg (5.3-110 mg/L). And their concentration in the treated effluents ranged from Na (130-800 mg/L), K (2.1-11.6 mg/L), Ca (8.18-1104 mg/L) and Mg (6.2-66.2 mg/L) (Table 1). The observed values indicated that Na and Ca concentrations both in the raw and treated effluents were much higher than the national and WHO discharge limits. The higher values of Na and Ca in the effluents were attributed to caustic treatment during the pulping process.
The heavy metals concentrations in the raw and treated effluent were within the National and WHO industrial effluents discharge limits (Table 1). However, higher level of Fe compared to other metals reflected the toxic nature of the pulp and paper mill effluents [14].
The correlation analysis results among selected physicochemical properties of raw and treated effluents were presented in table 2 and table 3. The analyses results for most parameters showed the expected trends in effluent quality.
| Parameter | BOD | COD | TN | Turb | TS | TDS | EC | PH | TH | Ca | Fe | Cl- | TA | CO32- | HCO- | SO42- |
| BOD | 1 | |||||||||||||||
| COD | 0.58 | 1 | ||||||||||||||
| TN | 0.96 | -0.77 | 1 | |||||||||||||
| Turb | -0.82 | 0.01 | -0.64 | 1 | ||||||||||||
| TS | 0.71 | -0.98* | 0.87 | -0.19 | 1 | |||||||||||
| TDS | 0.58 | -1 ** | 0.77 | -0.01 | 0.98* | 1 | ||||||||||
| EC | 0.81 | -0.94 | 0.94 | -0.34 | 0.99* | 0.94* | 1 | |||||||||
| PH | -0.09 | -0.76 | 0.18 | 0.64 | 0.63 | 0.76 | 0.50 | 1 | ||||||||
| TH | 0.98 | -0.42 | 0.90 | -0.91 | 0.58 | 0.42 | 0.70 | -0.27 | 1 | |||||||
| Ca | 0.98* | -0.41 | 0.89 | -0.92 | 0.56 | 0.41 | 0.69 | -0.28 | 1.0 | 1 | ||||||
| Fe | -0.10 | 0.87 | -0.36 | -0.48 | -0.77 | -0.87 | -0.66 | -0.98 | 0.08 | 0.10 | 1 | |||||
| Cl- | 0.43 | 0.98 | -0.65 | -0.16 | -0.94 | -0.98* | -0.87 | -0.86 | -0.26 | -0.24 | 0.94* | 1 | ||||
| TA | 0.81 | -0.94 | 0.94* | -0.34 | 0.99* | 0.94* | 1 ** | 0.50 | 0.70 | 0.69 | -0.66 | -0.87 | 1 | |||
| CO32- | -0.53 | -0.38 | -0.29 | 0.92 | 0.21 | 0.38 | 0.06 | 0.89 | -.68 | -0.69 | -0.79 | -0.54 | 0.06 | 1 | ||
| HCO3- | 0.94* | -0.82 | 1** | -0.58 | 0.91 | 0.82 | 0.96* | 0.26 | 0.86 | 0.85 | -0.44 | -0.71 | 0.96* | -0.21 | 1 | |
| SO42- | 0.45 | -0.99* | 0.67 | 0.14 | 0.95 | 0.99* | 0.88 | 0.85 | 0.28 | 0.27 | -0.93 | -1** | 0.88 | 0.52 | 0.73 | 1 |
Table 2: Correlation matrix (Pearson) of raw effluent.
*Correlation is significant at the 0.05 level (2-tailed)
**Correlation is significant at the 0.01 Level (2-tailed)
As shown in table 2, BOD5 of raw effluents correlated significantly positive with parameters such as TN (r=1), K (r=0.96), TH (r=0.98), Ca (r=0.98), COD (r=0.58) and HCO3- (r=0.94). However, it showed negative correlation with carbonates (r=-0.53), and Fe(r=-0.1), and turbidity(r=-0.82). This indicated that change in amount of TN, K, TH, Ca, COD and bicarbonates caused significant positive change in BOD5 of the effluents. TS and TDS (r=0.98) correlated significantly positive with each other as well as with EC (r=0.99), total alkalinity, sulphate, carbonate and bicarbonate. This confirmed that degradation of both TS and TDS reduced the dissolved oxygen of the effluents which led to an increase in the BOD5 and freely moving ions in the effluents.
As shown in table 3, total hardness of treated effluents had strong positive correlation with BOD5 (r=0.98), Ca (r=1), Mg (r=1). The COD of treated effluents had negative correlation with almost all parameters, including EC (r=-0.94), TS (r=-0.98), TDS (r=-1), TH (r=-0.42), HCO3 (r=-0.82), SO4-2 (r=-0.99) but positive correlation with turbidity (r=0.01), chloride (r=0.98), BOD5 (r=0.25) and Fe (r=0.87). This implied that the high value of conductivity, TS, TDS, BOD5 and TH increased COD. In general, the correlation analysis result in the treated effluent shown in Table 3 followed similar trends to raw effluents.
| Parameter. | BOD | COD | Turb | TS | TDS | EC | pH | TH | Ca | Fe | Cl- | TA | CO32- | HCO3- | SO42- |
| BOD | 1 | ||||||||||||||
| COD | 0.25 | 1 | |||||||||||||
| Turb. | -0.82 | -0.35 | 1 | ||||||||||||
| TS | 0.10 | -0.99 | 0.49 | 1 | |||||||||||
| TDS | 0.02 | -0.96 | 0.59 | 0.99 | 1 | ||||||||||
| EC | 0.37 | -0.99 | 0.22 | 0.96 | 0.92 | 1 | |||||||||
| pH | -0.73 | -0.48 | 0.99 | 0.61 | 0.70 | 0.37 | 1 | ||||||||
| TH | 0.93 | -0.60 | -0.55 | 0.47 | 0.36 | 0.69 | -0.42 | 1 | |||||||
| Ca | 0.93 | -0.60 | -0.55 | 0.47 | 0.36 | 0.70 | -0.41 | 1** | 1 | ||||||
| Fe | 0.99* | -0.18 | -0.86 | 0.02 | -0.10 | 0.30 | -0.78 | 0.90 | 0.89 | 1 | |||||
| Cl- | 0.30 | 0.85 | -0.79 | -0.92 | -0.96 | -0.77 | -0.87 | -0.08 | -0.08 | 0.37 | 1 | ||||
| TA | 0.21 | -0.99* | 0.38 | 0.99 | 0.97 | 0.99 | 0.52 | 0.56 | 0.57 | 0.14 | -0.87 | 1 | |||
| CO32- | -0.91 | -0.17 | 0.98 | 0.32 | 0.43 | 0.04 | 0.94 | -0.69 | -0.69 | -0.9 | -0.7 | 0.21 | 1 | ||
| HCO3- | 0.60 | -0.92 | -0.04 | 0.85 | 0.79 | 0.97 | 0.11 | 0.86 | 0.86 | 0.54 | -0.6 | 0.91 | -0.23 | 1 | |
| SO42- | 0.40 | 0.78 | -0.85 | -0.87 | -0.92 | -0.70 | -0.92 | 0.03 | 0.03 | 0.47 | 0.99 | -0.81 | -0.74 | -0.5 | 1 |
Table 3: Correlation matrix (Pearson) of treated effluent.
*Correlation is significant at the 0.05 level (2-tailed)
**Correlation is significant at the 0.01 Level (2-tailed)
Hierarchical Cluster Analysis (HCA) was used to classify water and effluent samples of the study area into different groups. The HCA was designed to group water samples based upon similarities in measured parameters. The squared Euclidian distance between values of parameters measured was used to calculate similarity among samples such that similar items could be agglomerated by using linkage methods [21]. Guler et al [22] described hierarchical cluster analysis as an efficient means to recognize groups of samples that had similar chemical and physical characteristics.
The dendrogram of hierarchical clustering (Figure 2) showed that there were two pairs of fairly close samples, [(C,E) and D], while the linkage distance between cluster (A,B) and others were far. This indicated samples D and (C, E) were more similar to each other than the samples (A,B). This difference in the sample cluster groups might be attributed to the distance from the source of effluents. Within each cluster the samples (C,E) or (A,B) showed more similarity, indicating composition similarities in measured parameters. Whereas, the least contaminated sample taken from upstream of the discharging point (D) separated itself entirely from the effluent samples (A,B), indicating that the effluents from the factory contributed to the change in composition of the river water samples taken downstream (C,E).
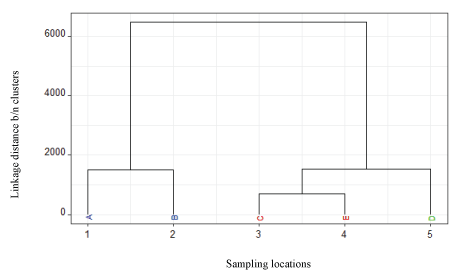
Figure 2: Dendrogram of hierarchical clustering of water samples from upper Awash River and effluent samples of the factory.
Spatial variations in the physicochemical characteristics of Awash River water as a result of effluents discharged from the factory were investigated by considering three sampling sites (Table 4). The pH of the upstream sampling point ranged from 7.1 to 7.9 with an average value of 7.5. The observed results were within the national maximum allowable limit of river water (Table 4). However, deviations in pH, at the factory discharge points (ranged from 5.1 to 9.4) with an average value of 7.3, and downstream of the river (ranged from 7.2 to 9.2) with an average value of 7.84, indicated significant effect of addition of the factory effluents on the river water pH. This change need frequent monitoring even though the current change was not expected to cause any adverse effect on the survival of aquatic organisms.
| Parameter | Mean ± SD | Maximum permissible concentration | |||
| Discharge point(C) | Downstream(E) | Upstream(D) | EEPAb | WHOa | |
| BOD (mg/L) | 429.19 ± 27.64 | 65.48 ± 3.18 | 6.81 ± 0.09 | 10 | 10 |
| COD (mg/L) | 1066.7 ± 76.01 | 314.37 ± 19.80 | 7.53 ± 0.45 | 40 | 40 |
| TP (mg/L) | 1.04 ± 0.01 | 0.82 ± 0.01 | 0.78 | 0.24 | 0.14 |
| TN (mg/L) | 9.06 ± 0.52 | 6.30 ± 0.18 | 2.40 ± 0.02 | 10 | |
| Turbidity (NTU) | 198.12 ± 13.5 | 129.58 ± 9.6 | 22.51 ± 1.4 | 5.0 | 5.0 |
| TS (mg/L) | 1048.67 ± 118.1 | 804 ± 92.79 | 327.33 ± 34.4 | ||
| TDS (mg/L) | 833.33 ± 68.77 | 663.3 ± 36.69 | 321 ± 13.57 | 500 | |
| TSS (mg/L) | 215.33 ± 13.55 | 140.67 ± 10.43 | 33.33 ± 2.44 | 100 | 80 |
| EC µS/cm | 1247 ± 12.6 | 1028.33 ± 10.3 | 469.33 ± 8.2 | 300 | 300 |
| pH | 7.23 ± 0.43 | 7.84 ± 0.44 | 7.50 ± 0.21 | 6.5-8.5 | 6.5-8.5 |
| Na (mg/L) | 279 ± 0.68 | 161.33 ± 0.87 | 28.50 ± 0.07 | 200 | 200 |
| K (mg/L) | 3.467 ± 0.1 | 3.93± 0.08 | 2.1 ± 0.02 | 1.5 | |
| TH (mg/L) | 363.09 ± 30.8 | 345.08 ± 28.65 | 28.20 ± 1.5 | 500 | |
| Ca (mg/L) | 118.33 ± 6.7 | 121.43 ± 5.12 | 74.62 ± 3.18 | 75 | 75 |
| Mg (mg/L) | 16.13 ± 0.92 | 9.96 ± 0.76 | 7.63 ± 0.49 | 50 | 50 |
| Cl− (mg/L) | 97.47 ± 4.76 | 50.10 ± 1.34 | 5.99 ± 0.27 | 600 | |
| Total Alkalinity (mg/L) | 428.54 ± 29.42 | 353.01 ± 24.5 | 237.35 ± 16.2 | 200 | 200 |
| CO32− (mg/L) | 19.59 ± 0.62 | 29.39 ± 1.31 | ND | ND | ND |
| HCO3− (mg/L) | 482.80 ± 19.2 | 370.91 ± 17.34 | 289.6 ± 11.2 | - | - |
| SO42− (mg/L) | 56.3 ± 0.95 | 194.65 ± 1.84 | 4.89 ± 0.03 | 250 | 250 |
| Cu (mg/L) | ND | 0.01 | 0.03 | 2 | 1.5 |
| Zn (mg/L) | 0.06 | 0.07 | 0.09 | 5 | 5 |
| Fe (mg/L) | 0.29 | 0.38 | 0.13 | 0.3 | 0.3 |
Table 4: Average (n=3) concentrations of selected physicochemical parameters of upper Awash River compared with river water maximum allowable standard concentrations.
aWHO (2008) World Health Organization standard limit
bEEPA (2010) Ethiopian Environmental Protection Authority
Chemical parameters classification by the use of hierarchical cluster analysis shown in figure 3 indicated correlation among measured parameters and the relative contribution of measured physicochemical parameters from the factory effluent on the river water composition.
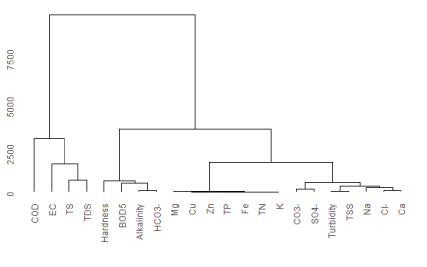
Figure 3: Dendrogram of hierarchical clustering of water samples from upper Awash River and effluent samples of the factory.
The mean Electrical Conductivity (EC) of Awash River water at the effluent discharge point (C), downstream point (E) and upstreampoint (D) were 1247, 1028, and 469 µS/cm, respectively. The EC values obtained at all sampling points exceed the national [23] and WHO maximum permissible limits of 300 µS/cm. Higher EC value were observed at C and D (means samples taken after effluent discharge) as compared to upstream value (Figure 4). The higher EC at discharge and downstream points are attributed to the discharge of effluents from the factory into the river. Conductivity in water analysis is used to indicate the contents of dissolved solids in the river water and found to be unsafe to aquatic live as the observed value is above the permissible limit [24].
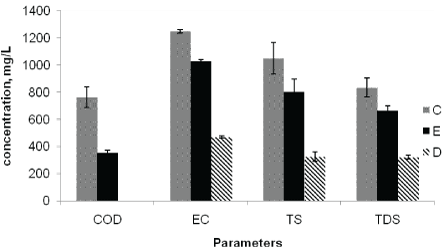
Figure 4: Average concentrations of COD, EC, TS and TDS at the point of discharge (C), upstream of the effluent entry point (D), and downstream of the effluent entry point (E).
The highest COD values were recorded at the effluent discharging point (1066 mg/L), and the lowest (7.5 mg/L) at the upstream points (Figure 4). This indicated the presence of high oxygen demanding organic pollutants and inorganic pollutants, such as sulphites in the discharged effluents whose oxidation also demands oxygen [7].
The mean concentration of Total Solids (TS) at sampling points C, E and D of the river water were 1048, 804, and 327 mg/L, respectively (Table 4). The lowest TS was found in the upstream points (nonpolluted samples), whereas the observed result after the factory effluent discharge showed the TS were higher at the discharge point than downstream points (Figure 4). Similarly, Total Dissolved Solids (TDS) values were higher at point of discharge (833 mg/L) followed by downstream points (663 mg/L) than upstream point (321 mg/L) (Figure 4). The observed values of TDS after the factory effluent discharge were higher than the WHO maximum permissible limit of 500 mg/L for surface water.
The mean Total Suspended Solids (TSS) contents of the river water at sampling points C, E and D were 215, 140, and 33 mg/L, respectively. The TSS values were higher at the discharge point than the other sampling points followed by downstream samples contained more TSS than the upstream samples (Figure 5). The TSS values obtained at discharge and downstream points were higher than the WHO maximum limit of 100 mg/L for surface water. The mean turbidity values of the upper Awash River water samples varied significantly among the different sampling points C (198 NTU), E (122) and D (22.5 NTU) (Figure 5). The turbidity values obtained at all locations were higher than the National [23] and WHO standard limits of 5 NTU for river water. The results clearly showed the excessive turbidity, TDS, TS and TSS in the upper Awash River water is associated with the factory effluent addition. This might reduce the aesthetic value of the river and decrease the photosynthesis process by preventing deep penetration of light into the water [25].
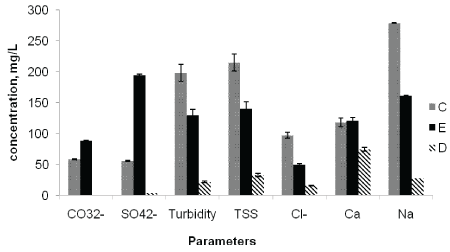
Figure 5: Average concentrations of CO32-, SO42-, Turbidity, TSS, Cl-, Ca and Na at the point of discharge (C), upstream of the effluent entry point (D), and downstream of the effluent entry point (E).
The BOD5 values measured at sampling points C, E and D were 429, 65.5 and 6.8 mg/L, respectively. The highest BOD5 values were observed at the effluent discharge point and the lowest values at the upstream point (Figure 6). This indicated the discharged effluents from the factory contained high organic matter content that affect the river water quality. The observed BOD5 and COD levels (Table 4) were also found to be above the National [23] and WHO maximum allowable limit of river water of less than 10 mg/L and 40 mg/L, respectively.
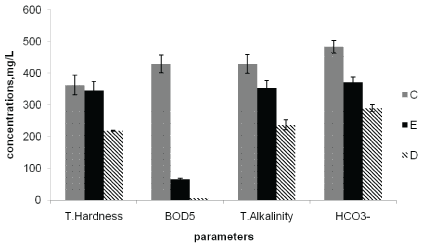
Figure 6: Average concentrations of Total Hardness, BOD5 , Total Alkalinity and HCO3- at the point of discharge (C), upstream of the effluent entry point (D), and downstream of the effluent entry point (E).
The mean values of total alkalinity were highest at effluent discharge point (428 mg/L) followed by downstream sampling point (353 mg/L) and the lowest value recorded at the upstream point (237 mg/L). The total alkalinity found in all sampling points were in excess of the National (EEPA, 2010) [26] and WHO maximum allowable limit of 200 mg/L of surface water (Table 4). The mean bicarbonate content also showed similar trend in which the highest value was obtained at the downstream sampling point (483 mg/L) and the lowest at the upstream sampling point (289 mg/L) (Table 4). This resulted from the nature of the factory effluents discharged into the river [4].
The mean values of total hardness at sampling points C, E and D were 363, 345, and 28.5 mg/L, respectively (Table 4). The values at the discharge point and downstream point were above the WHO maximum allowable limit of 500 mg/L. On the bases of total water hardness [27], samples can be classified as soft (0-70), moderately soft (75-100 mg/L), moderately hard (100-150 mg/L), Hard (150-300 mg/L), Very hard (above 300 mg/L). As a result the observed value in Awash river water samples collected after mixing the effluent were found in the range of very hard water. This is attributed to the addition of effluents from the factory which contained much dissolved calcium and magnesium ions. An increase in hardness level adversely affects detergent performance which constitutes the major problem to the human population who rely on the surface water for cleaning purpose [28].
The mean total phosphorus (TP) load at sampling points C, E and D were 1.04, 0.82, and 0.78 mg/L (Table 4). The observed values were in excess of the WHO permissible limit of 0.14 mg/L and would cause eutrophication. The elevated TP levels at all sampling sites could possibly result from soap and detergent used by the nearby population used the river for bathing and discharge their domestic effluents besides of effluent discharge from the factory. Similar trends were also observed with respect to TN and the mean TN values at sampling points C, E and D were 9.06, 6.30, and 2.40mg/L, respectively (Table 4).
The mean concentration of chloride at sampling points C, E and D were 97.5, 50.1 and 5.99 mg/L, respectively (Table 4). Chlorides in water in general are not harmful to human and other animals up to a concentration of 600 mg/L [29]. However, pulp and paper effluents containing chlorine compounds may couple with high organic content results in the production of highly toxic organic compounds which are known to be persistent and bio-accumulating. Hence, cause harmful disturbances in biological systems, at the same time posing human risk through long term exposure [10,11].
The mean SO42- concentration at sampling points C, E and D were 56.3, 194 and 4.89 mg/L, respectively (Table 4). Unlike other parameters, the observed trend with respect to SO42- showed higher level at downstream point than at the effluent discharge point. This might be resulted from the higher BOD5 and COD values at the discharge point than downstream point accompanied with the lower level of dissolved oxygen that resulting in the subsequent reduction of sulfate to sulfide at the discharge point [26]. However, the increased concentration of sulfate at the downstream site is attributed to the decrease in organic load resulting in the oxidation of sulfides to sulfate.
The mean concentration of Mg at the sampling points C, E and D were 16.1, 9.96 and 7.63 mg/L, respectively. And the mean Ca concentrations were 118, 121, and 74.6 mg/L, respectively (Table 4). The highest concentrations of Mg and Ca were observed at the effluent discharge point followed by downstream points, and the lowest value was recorded at upstream point. The concentration of Ca at the three sampling sites were above the WHO maximum limit of 75 mg/L for surface water, while the concentration of Mg was found below the recommended limit of 50 mg/L.
The mean concentration of sodium at sampling points C, E and D were 279, 161 and 28.5 mg/L, respectively. And the mean concentrations with respect to potassium were 3.47, 3.93, and 2.10 mg/L, respectively (Table 4). Higher concentrations of both K and Na were obtained at the effluent discharge point than downstream points. The lowest values of K and Na were obtained at the upstream sampling point. This clearly indicated the factory effluent contained higher level of Na and K probably from the caustic treatment during pulping process.
The mean values of heavy metals analyzed at the sampling points C, E and D were within the national and WHO (2008) [23,29] standards (Table 4). However, the concentration of Fe was slightly higher at downstream of the river (0.38 mg/L) which reflects the influence of the factory effluents in the river.
Download Provisional PDF Here
Article Type: RESEARCH ARTICLE
Citation: Jebessa ZF, Wondemagegnehu EB (2018) Physicochemical Characterization of Upper Awash River of Ethiopia Polluted by Anmol Product Paper Factory. Int J Water Wastewater Treat 4(2): dx.doi.org/10.16966/2381-5299.154
Copyright: © 2018 Jebessa ZF, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Publication history:
All Sci Forschen Journals are Open Access