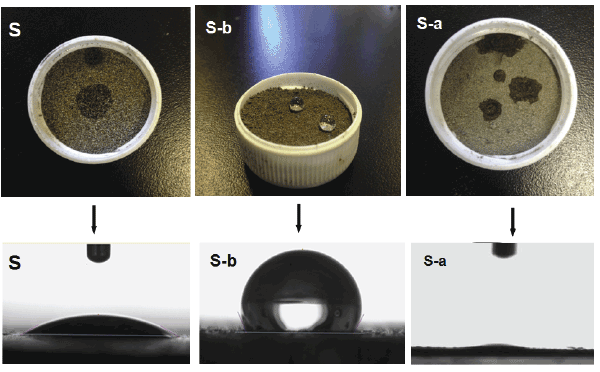
Figure 1:Surface wetting behaviors and images of the droplets on the sample of S, S-a and S-b.


Xingjian Jiang1 Fanqing Meng C2 Wei Ma*,2
1Shenhua Yulin energy chemical CO.LTD, Yulin city 719302,Shanxi province, P.R.China*Corresponding author: Wei Ma, Research Centre of Seawater Desalination and Multipurpose Utilization, Department of Chemistry, Dalian University of Technology, Dalian 610023, P.R. China, E-mail: mawei@dlut.edu.cn
Ammonia-nitrogen is the predominant pollutant in the main drinking water sources, which is also, can cause failure in water treatment process due to the inhibition effect. Our study confirmed that the freeze separation process seems to have potential for ammonia removal from water. The effect of different operational factors including initial concentration of ammonia and ion species were also investigated, wherein the inhibition is ordered as follows: Mg2+>Ca2+>Na+>K+. Experimental data have shown that the surface modification of the nucleating agent plays a very crucial role in ice nucleation and the process obtained a ammonia removal efficiency as high as 93, 89 and 85% for the S-a, S and S-b spiked with 450 mg/L ammonia, respectively. In addition, the results also show that those four solutions have different ammonium removal speed and the ordered are as follows: S-a>S>S-b> only water aqueous solution. Moreover, the date also shows that this method reveals highly effective to remove ammonia in the real ground water. The mechanism study reveals that hydrophilic material became nucleation centers and promoted the freezing process. The hydrophobic material solution needs more time to freezing due to decreased of the freezing temperature.
Ice nucleation; Ammonia removal; Freezing temperature; Freeze desalination; Hydrophobic and hydrophilic modification
Ammonia-nitrogen, including non-ionized (NH3) and ionized (NH4+), is the predominant pollutant in the main drinking water sources [1]. It originates from human activities in the urban areas, metabolic, agricultural and industrial processes, and from disinfection with chloramine [2]. Ammonia-nitrogen concentration in surface water is affected by hydrogeology and climate change [3-5]. Ammonia toxicity in waters has been widely investigated: its incomplete nitrification elevates toxic nitrite contents; due to its property to combine chlorine easily, the chlorine necessity during disinfection processes increases, and consequently the content of mutagenic disinfection by-products (DBP) is changing in an ascending mode [6-10]. It also causes the failure of filters for the removal of manganese, and causes taste and odour problems. Extremely high ammonia concentrations (1500–5000 mg/L) can cause failure in wastewater treatment process due to the inhibition effect [11,12]. In China, the direct drainage standard of the ammonia is 10 mg/L, therefore, there is a great demand of finding a low cost, and high efficiency method for the removal of ammonia from aqueous solution to protecting environment and human health.
Conventional methods for ammonia removal from aqueous solution include biological treatment, air stripping, ion-exchange and adsorption [13]. Traditional biological processes incorporate nitrification and denitrification and they do not perform well to the shock ammonia loading [14,15]. Air stripping provides satisfactory results in high pH conditions where most ammonia is in unionized form (NH3) [16]. Chemical precipitation needs additional reagents, which may introduce new pollutants, while electrochemical method often uses expensive metal as electrodes and also consumes large quantity of energy [17]. Ion-exchange and adsorption processes have received more interests as possible treatments due to relatively simply operation, however, recent study has proven that tedious separation process of the exhausted powdered adsorbent also has some insufficient such as the effluent and the regeneration/disposal limitation [18]. Finding efficient and low-cost method for ammonia removal has become one of the research focuses in the field of ammonia removal from liquid phase.
Freezing and subsequent removal and melting of the ice is an alternative physical process which can be used for desalting, based on the different freezing points of fresh and salt waters. It has been reported as being effective to remove various organic and inorganic impurities from water/wastewater [19,20]. When freeze concentration is used to purify water or liquid waste, impurities are separated from the ice phase during formation of the ice crystals. Two basic freeze desalination methods are available: suspension and progressive freeze crystallization [21]. In both processes, inclusion of most compounds in the ice crystal lattice is impossible due to the small dimensions of ice crystal lattice. In progressive freeze crystallization, the separation of ice crystals formed from the concentrated mother liquor is much easier than in the conventional suspension crystallization, in which many small ice crystals are formed. In theory, freeze desalination has a lower energy requirement compared to other thermal processes. Recently, Fekadu Melak et al. [22] focused on assessing the potential of home-use refrigerators to generate drinking water from Cr-contaminated water.
However, there are few references were found to removal ammonia by freezing process. The study of effect of the surface of the nucleation catalyst on the freeze separation process has been little investigated. Therefore, the main objective of this study was to removal of ammonia from aqueous solution by freeze separation process. The effects of different operational factors including initial concentration of ammonia and ion species were also investigated. In addition, the effect of modified surface of the nuclear material on freezing process and icing mechanism were been studied.
An stable alumino silicate (S) which was purchased from huangming company of China, N,N-methylenebisacrylamide (MBA) and K2 S2 O8 were obtained from Sinopharm Chemical 71 Reagent Co., China. Stearic acid (C18H36O2 ), acrylic acid (C3 H4 O2 ) and other reagents used in this work were of analytical grade and purchased from China. All the reagents used in the study were analytical grade, and all solutions were prepared with deionized water
The water-retaining fertilizer was synthesized as follows: stable alumino silicate (4 g) were washed by deionized water then washed by ethyl alcohol and dried at 60°C with N2. Next, the product was dispersed into 20 mL deionized water, and then 5 g Stearic acid, 0.2512 g K2 S2 O8 and 0.1880 g MBA were added in the flask. The mixture was irradiated in the microwave oven at 300 W for 30 s. The resulting gel product was dried at 50°C and sifted through a 50-mesh sieve which was been named S-a.
Firstly, 4 g stable alumino silicate (S) were washed twice times by deionized water then washed by ethyl alcohol and dried at 60°C with N2. Then, the product dispersed into 100 mL deionized water and reacting for 30 min at 30°C. Next, 2 g melted stearic acid was been added into the mixed solution. The solution was left for reacting for 60 min at 30°C. Then the resultant samples were washed with deionized water followed dried with N2 and sifted through a 50-mesh sieve which was been named S-b.
The anti-icing test was conducted in refrigerator environmental to ensure the stability of the refrigeration temperature. Firstly, the samples were placed into the same ammonium solution (500 mg/L). Then the rapid cooling process was carried out with the stable temperature at -20°C by using the temperature control refrigerator (DW-COOL362, China, Shanghai), the surface of tested sample was recorded with a microscope camera, the liquid was removed by a injection syringe and the concentration of the ammonia is analysis by the UV spectrophotometer (Zhongkeda Dalain, China).
The surface wetting behaviors were obtained on a contact angle measurement instrument (JC2000D2, Shanghai Zhongchen Digital Technology Apparatus Co., Ltd) with a 2 µL deionized water droplet. Fourier transform infrared (FTIR, Nicolet IS-10) spectroscopic studies were performed using spectroscopy in 500-4000 cm-1 range. Spectrophotometric method was used to measure ammonia.
Use formula 1 to calculate the desalting efficiency:
\[Removal - rate(\% ) = \frac{{{C_0} - {C_5}}}{{{C_0}}} \times 100\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,(1)\]
where, Co and Cs are the ammonia concentrations in the initial feed solution and the remaining ice phase, respectively
The images of S, S-a and of S-b are presented in figure 1. The volume of the individual droplet is 2 µL. As can be seen, surface with water contact angle (CA) of S-a is about 4° and S-b show very exhibited high CA values about 145°. The wetting behaviors could be ascribed to the following two categories: surface chemical compositions and surface structures.

Figure 1:Surface wetting behaviors and images of the droplets on the sample of S, S-a and S-b.
The surface functional groups were determined by the FTIR spectra and the results were displayed in figure 2. The special peaks of S-a around 3400 and 1670 cm-1 are mainly due to the O-H and C=O bonds, indicating the existence of acrylic acid. Therefore, the surfaces of S-a is intrinsically hydrophilic. Characteristic peaks of S-b around 2950 and 1500 cm-1 are due to the stretching and bending of C-H bonds [23], suggesting the successful grafting of stearic acid on steel surface and revealed hydrophobic property.
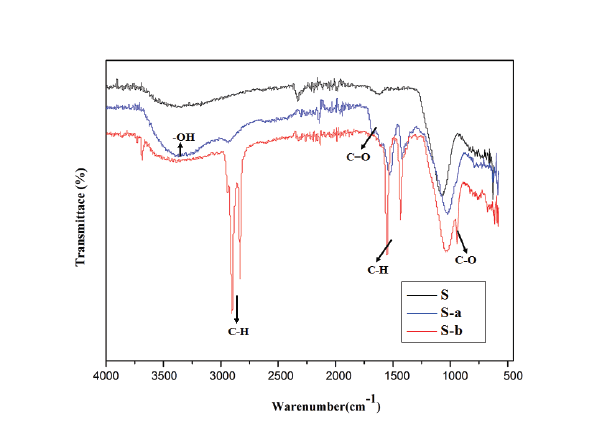
Figure 2: FTIR spectra of the three prepared materials.
The effect of initial concentration of ammonium ions on the rate of icing form and the removal rate of ammonium are presented in figure 3. As is shown in the picture, the efficiency of the icing form and the collecting of ammonium in the aqueous solution were decreased sharply when the initial ammonium concentration increased from 450 to 1500 mg/L. However, there was no significant decrease on both of them with increase of ammonium concentration from 100 to 450 mg/L. What’s more, the biggest removal rate of ammonium appeared when the ammonium concentration up to 450 mg/L. Therefore 450 mg/L was chosen as the optimum initial concentration for ammonium ions beneficiation.
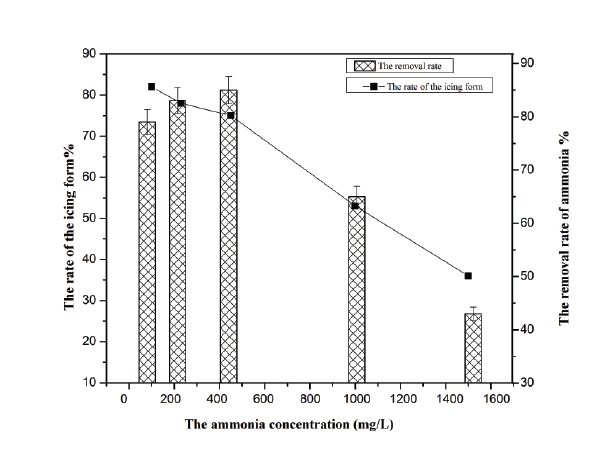
Figure 3: The effects of ion species on the removal rate of the freezing process.
Figure 4 presented the removal rate of the ammonium and the rate of icing form employed in the experiment for simulation decreased in the order: K+>Na+>Ca2+>Mg2+ and the concentration of those ions are 10 mg/L. This might be affect by the magnitude of hydration free energy for the studied ions is provided in the order: Mg2+>Ca2+> Na+>K+. Ions in water are found in hydrated forms which can be described as Mn+ (H2 O)n , with n water molecules coordinated with the cation in a geometrically defined hydration shell. Hydration free energy shows the stability of the hydrated ions in reference to their unhydrated counterpart. Ions having a strong interaction with water molecules are more easily incorporated in the ice phase during freezing. Thus, ions with smaller energy of hydration have less association with water and hence high removal percentage [24].
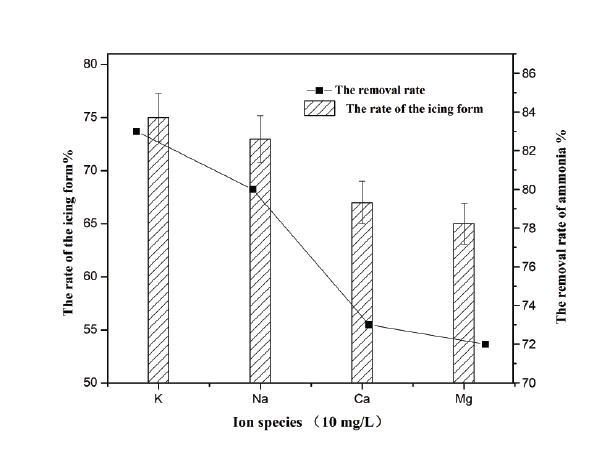
Figure 4: Effect of initial concentration of ammonium on ammonium removal.
As can be seen in figure 5, three materials have similar the general tendency of the rate of ice growth and the removals rate of ammonium ions in the aqueous solution. But an effect of three materials on the ice growing rate was different and the ordered are as follows: S-a>S>S-b. Experimental data and literature search results have shown that the surface plays a very crucial role in ice nucleation [25]. The results shows that both the rate of ice growth and the removal rate of ammonium ions are higher on the S-a solution and lower in the S-b solution. To understand these results we need to analyze these data in combination with the ice morphology and the distribution of the nucleation centers in the ammonium solution.
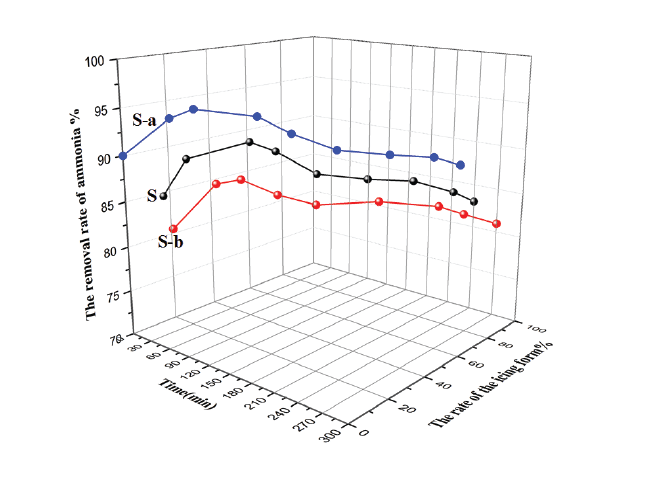
Figure 5: Effect of modified surface of the nuclear material on freezing process and ammonium removal rate.
The ammonium concentrations of the final products in the four solutions (S-a, S, S-b and water aqueous solution) were analyzed and the results were illustrated in figure 6. As the data shows that the S-a solution have the lower final ammonium concentration of 8 mg/L when the frozen time up to 80 min. The ammonium aqueous solution without any additive reveals the higher final ammonium concentration which is four times bigger than the direct drainage standard of the ammonia of China. What’s more, the results also show that those four solutions have different ammonium removal speed and the ordered are as follows: S-a>S>S-b> only water aqueous solution. This may because the S, S-a and S-b have important properties which can enhance the ammonium removal process.
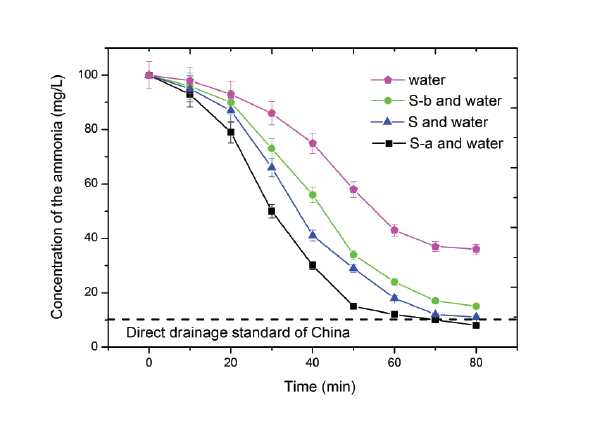
Figure 6: The ammonium concentration of the final products in the S-a solution, S solution, S-b solution and only water aqueous solution.
Finally this method was used to remove ammonium the real ground water solution. The real ground waters are more complicated relative to the synthetic solutions. For example, various kinds of salts, organic matters, and even microbes might be present in real waters. In this study, reservoir source water from China Dalian was used to represent the real water. The reservoir source water had a high conductivity (493 S/cm). The measured pH and COD of the ground water was 8.23 and 16 mg/L, respectively. To simulate the removal rate of the ammonium by the freezing process, three powders were mixed with 100 mL the reservoir source water solution which internal concentration is 81.3 mg/L. The final ammonium concentrations of the four solutions (S-a, S, S-b and water aqueous solution) were analyzed after the freezing time up to 80 min. The results show that final ammonium concentration of the four solutions was 9.8, 14, 19 and 27 mg/L, respectively. It is indicating that this method could work as a highly effective method for the remove of ammonium in the real solution.
Figure 7 displays the images of S, S-a and S-b in the icing test. It is obvious that the surface of S was changed after hydrophilic modification and hydrophobic modification process. What’s more, S-a and S-b showed different effects during the early stages of ice formation. As observed in figure 7, the hydrophilic modified material S-a became nucleation centers in the freezing process, but the hydrophobic modification material always on the surface of the ice during the freezing process. It is indicated that the wettability characteristics of the hydrophilic surfaces lead to a higher nucleation rate of nucleation centers during the early stages of ice formation. Therefore, during the early stages of ice formation, both the rate of ice growth and the removal rate of ammonium ions are higher on the hydrophilic solution which to be another support to figure 5. The delayed freezing process of the S-b could be ascribed to the lower freezing point of water on the hydrophobic surface, which could be explained by the Clapeyron relationship [26]. The surface of prepared hydrophobic S-b is comprised of micro−nano hierarchical structures, which means capillaries structures were been formed. The formula is shown below:
\[{{\rm{P}}_{\rm{r}}}{\rm{ = }}{{\rm{P}}_{{\rm{exp}}}}{\rm{(2r}}{{\rm{V}}_{\rm{m}}}{\rm{/R}}{{\rm{T}}_{\rm{r}}}{\rm{)}}\,\,\,\,\,\,\,\,\,\,\,\,\,{\rm{(2)}}\]
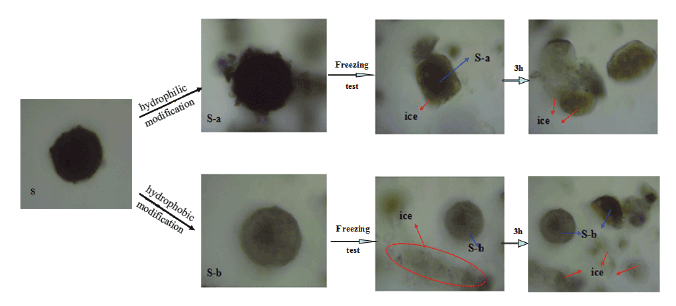
Figure 7: The picture of the ice morphology formed on differently treated surfaces of S-a and S-b at the freezing process.
where Pr and P are the vapor pressure and saturated vapor pressure at a certain temperature, Vm is the molar volume of the phase, γ is the surface energy, R is the gas constant, T is the temperature, and r is the radius of the droplet or crystal. As we all know, the small droplets with high vapor pressure is not easy to contain, thus S-b reveals the hydrophobic property. For solidification process, the Clapeyron relationship could be transformed as:
\[\frac{{dT}}{T} = \frac{{{\Delta _s}{V_m}}}{{{\Delta _s}{H_m}}}dp\,\,\,\,\,\,\,\,\,\,\,\,\,(3)\]
where P is the vapor pressure, T is the temperature; ∆s Vm and ∆s Hm are the volume difference and enthalpy change during the solidification process, respectively. After integration of equations 2 and 3, we get the following equation:
\[1n\frac{{{T_2}}}{{{T_1}}} = \frac{{{\Delta _s}{V_m}}}{{{\Delta _s}{H_m}}}({P_2} - {P_1})\,\,\,\,\,\,\,\,\,\,(4)\]
During the freezing process, the ∆s Vm>0, ∆s Hm<0, thus, the T is inversely proportional to P. For the condensed water on S-b surface, the lower the freezing temperature would be obtained due to the smaller r of droplet on the hydrophobic surface [27]. Therefore, compared with the bare substrate of S, condensed water on the hydrophobic surface of S-b tends to be super cooled, and the freezing temperature of water decreased largely, thus when put into a cold environment along with the water, the hydrophobic material solution take more time to freezing.
This study confirmed that the freezing separation process have high potential for the removal of ammonia from water. The effects of different operational factors including initial concentration of ammonia and ion species were investigated. Where in the inhibition is ordered as follows: Mg2+>Ca2+>Na+>K+. In addition, the results also show that those four solutions have different ammonium removal speed and the ordered are as follows: S-a>S>S-b> only water aqueous solution. The process obtained an ammonia removal efficiency as high as 93, 89 and 85% for the S-a, S and S-b spiked with 450 mg/L ammonia, respectively. Moreover, the date also shows that this method reveals highly effective to remove ammonia in the real ground water. Experimental data have shown that the surface of nucleating agent plays a very crucial role in ice nucleation because of their hydrophilic and hydrophobic property [27]. Moreover, the mechanism studies shows that hydrophilic material became nucleation centers and promoted the freezing process, but hydrophobic material tends to be decreased the freezing temperature of water and prolong time to freezing.
Financial supports from the Chinese National Nature Science Funding of China (U1403194) are gratefully acknowledged.
Financial supports from the National High Technology Research and Development Program (‘‘863’’ program) of China (2012AA06A115) are gratefully acknowledged.
Download Provisional PDF Here
Article Type: Research Article
Citation: Jiang X, Meng F, Ma W (2016) The Effect of Surface Modification on Removal of Ammonia by using Ice Formation. Int J Water Wastewater Treat 2(5): doi http://dx.doi.org/10.16966/2381-5299.130
Copyright: © 2016 Jiang X, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Publication history:
All Sci Forschen Journals are Open Access