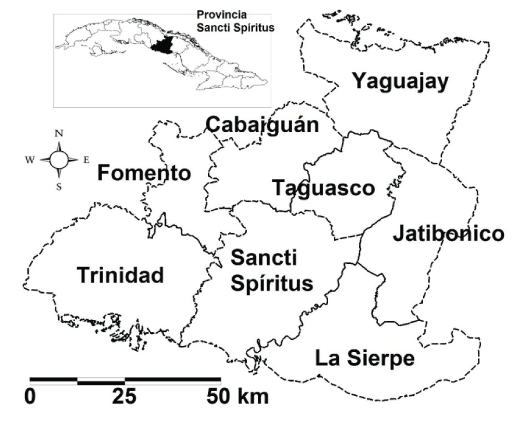
Figure 1: Localization of La Sierpe in Sancti Spíritus province, Cuba

Maritza Pupo-Antunez1* Yaimee Vázquez2 Maya Andonova3 Susana Vázquez4 Luis Morier1 Mayda Castex4 Pedro Blanco5 Bárbara Sánchez5 Carlos Cruz6 Guzmán MG4
1Microbiology Department, Virology Lab, Biology Faculty, University of Havana, Cuba*Corresponding author: Maritza Pupo-Antunez, Microbiology Department, Virology Lab, Biology Faculty, University of Havana, Cuba, Tel: +53- 7-832 92 52; E-mail: mpupo@fbio.uh.cu
West Nile virus has circulated in many countries in Latin America after 1999; however, no outbreaks have been reported and in most of them, the virus has not been isolated yet. In Cuba, its circulation was documented since 2005 but the isolation of the virus has not been succeeded. A field study was performed to isolate the virus from mosquitoes and to detect flavi virus and West Nile virus antibodies in humans and birds. The study was conducted 2 localities in La Sierpe municipality, Sancti Spíritus province where the first West Nile virus human cases were reported in 2006. Three hundred and four human sera samples, 391 adult birds (32 species) and 234 pools of mosquitoes (9 species) were collected from both localities. In human sera samples, 60% of them were positive to Dengue 2 and 0.65% was positive to West Nile virus. In birds, the 14.4% of terrestrial and 61% of aquatic birds had flavivirus antibodies; meanwhile 1.4% from terrestrial and 19.4% of aquatic were specific to West Nile virus. The most representative species of mosquitoes captured was Culex quinquefasciatus. West Nile virus was not isolated neither detected by molecular methods. However, some of these pools were positive by RT-PCR to flavivirus, suggesting the presence of an insect flavivirus.
Flavivirus; Mosquitoes; Birds; Antibodies; Cuba
Mosquito-transmitted viruses are a cause of public and veterinary health problem around the world. The majority of them are transmitted by hematophagous arthropod vectors, belonging mainly to three genera: Flavivirus (family Flaviviridae), Alphavirus (family Togaviridae), and Orthobunyavirus (family Bunyaviridae) [1]. However there is a number of flavivirus which apparently are Insect-Specific (ISVs) [2]. These flaviviruses include cell fusion agent virus, Kamiti River, Culex flavivirus and Quang Binh virus among others [3].
West Nile Virus (WNV) is a mosquito-transmitted flavivirus involving in its transmission cycle a diversity of wild birds and species of mosquitoes mainly Culex. Humans and most other nonavian species are incidental hosts, due to that they are not capable to produce viremias of sufficient degree to infect susceptible mosquitoes.
After its emergence in New York City in 1999, WNV spread from North America to Caribbean and South America. Until now, despite of this dissemination in Latin America no outbreaks have been reported and the isolation of WNV has been reported just in some of them [4-7].
In Cuba, WNV circulation was documented since 2005 [8] but the virus has not been isolated yet. In this study, we performed an entomological, ornithological and serological investigation in order to know the WNV activity and try to isolate the virus in mosquitoes to characterize it genetically and phylogenetically. The field study was conducted in 2 localities La Sierpe and El Jibaro; both appertaining to La Sierpe municipality from Sancti Spíritus province where the first human cases were detected.
La Sierpe has as topographical characteristics of its terrestrial that is composed by winding waterways and roads which cross the region. There are not bays, gulfs or capes and it is a short swampy close to the littoral with a predominant flat border. It has a media temperature about 25 and 27 Celsius degrees and a rain media annual of 1200 mm in the south and 1500 mm in the north. These conditions attract many bird species from Anseriformes, Charadriformes, Ciconiformes and Gruiformes orders and mosquitoes abundance [9]. The area selected for the field studies were 2 localities La Sierpe and El Jibaro; both appertaining to La Sierpe municipality (Figure 1). From this municipality were selected 8 capturing areas (P5, La Sierpe, El Jíbaro, Cementerio La Sierpe, El Almendrón, Granja Botijuela, Sur y, Granja Pitajones). The sampling sites were selected taking into account the presence of backyard poultry, and safe vehicular access.

Figure 1: Localization of La Sierpe in Sancti Spíritus province, Cuba
From localities La Sierpe and El Jibaro were taken of 137 and 167 human samples respectively. Sera were eluted in PBS/antibiotic from filter paper, dilution 1/20 in order to detect IgG Den and WNV antibodies using an ELISA inhibition method (EIM, and ELISA kits (Focus Technologies, Cypress, CA, USA).
The EIM to detect IgG anti dengue was followed as previously described [10,11]. Briefly, polystyrene plates (Costar No. 3591) were adsorbed with human anti-dengue IgG; after blocking, dengue 2 antigens previously diluted 1/40 in PBS plus 0.05% Tween-20 was added in each well. Serum samples diluted from 1/20 to 1/40 960 were tested. Volumes of 100 µL of each sample were added. Human IgG anti-dengue peroxidase conjugate diluted 1/3000 in PBS plus 0.05% Tween-20 and 2% fetal bovine serum were added. Substrate containing OPD was added. The reaction was stopped after 30 minutes incubation. The test was read at 492 nm. The inhibition percent was calculated as:
Inhibition%=[1-(OD sample/OD negative control)] × 100
The antibody titer for each sample was considered as the highest dilution with a percentage of inhibition ≥ 50.
Human sera were screened for WNV using IgG by using Focus Technologies according to manufacturer’s instructions. Briefly, samples were diluted 1:101 in Sample Diluent. Wells were soaked with Wash Buffer solution 1 × for 5 min after this time the plate was decanted. Hundred micro liters of samples and controls were added for 60 min. Plates were washed 3 times and 100 µL of peroxidase-conjugated goat anti-human IgG put into each well. The wash was repeated and 100 µL of substrate reagents were added during 10 min. The reaction was stopped with 100 µL of Stop Reagents and the lecture was performed at λ=450 nm. Calculation was done following the instructions of West Nile virus Dx SelectTM (FOCUS Diagnostics, Code EL0300G).
Reactive serum samples were further tested by a Plaque Reduction Neutralization Test (PRNT) with WNV (NY99, Ontario, Canada, 2001 isolate), SLEV (Saint Louis Encephalitis Virus, Parton strain, American Type Culture Collection catalog no: VR-1265) and dengue virus (dengue 2, NG-C strain). PRNT was performed as described previously [8]. Samples were considered seropositive if the 90% PRNT titer for that virus was >4-fold greater than the neutralization titers determined for other viruses used in the assay. Endpoint titrations were defined as the highest dilution of serum that reduced plaque formation by >90%.
Terrestrial birds were captured with 9 m mist nets which were placed from 7:30 am to 6:30 pm during a week. Serum samples were taken using Syringes (1 cc) and needles (23 gauges) for jugular or wing vein and collected in 1.5 ml Sarstedt vials or absorbed in filter paper (Nobutu) (volume governed by body weight). After taking the blood sample the birds were let out. Several data like date, sample locality, bird’s species, sex, relative age and kind of sample were registered. Aquatic birds were captured with shotgun for 1-2 days per locality.
Determination of antibodies to flavivirus and specifics to WNV by blocking Enzyme Linked Immunosorbent Assay (b-ELISA).
Nobutu filter paper with sera samples adsorbed in were cut and diluted in Phosphate Buffered Saline (PBS) and 1% antibiotic at 1/10 dilution and kept at 40°C overnight.
Two Mabs were used 3.1112G WNV (subtype KUNV, Chemicon International Product Code MAB8152) to detect specific WNV antibodies and 4G2 to detect flavivirus antibodies (CDC Catalogue Number: VS2378).
The b-ELISA was performed according to Blitchiv [12] with some modifications and WNV b-ELISA SOP 2008-1 Zoonotic Diseases and Special Pathogens National Microbiology Laboratory Public Health Agency of Canada.
Ninety six well micro titer plates (Costar No. 3591) were coated with 100 µL WNV NY 99 antigen of suckling mouse brain at 1/100 dilution and as control antigen was used suckling mouse normal brain at 1/250 dilution in carbonate-bicarbonate buffer (50 mM sodium carbonate, 50 mM sodium bicarbonate, pH 9.6). Coated plates were sealed individually and were incubated at 40°C overnight. Next day, plates were washed 5 times with 300 µL of PBS with 0.1% of Tween 20 (PBS-T) and 200 µL blocking buffer (PBS containing 5% skim milk) The b-ELISA was performed according to Blitchiv [12] with some modifications and WNV b-ELISA SOP 2008-1 Zoonotic Diseases and Special Pathogens National Microbiology Laboratory Public Health Agency of Canada.
Ninety six well micro titer plates (Costar No. 3591) were coated with 100 µL WNV NY 99 antigen of suckling mouse brain at 1/100 dilution and as control antigen was used suckling mouse normal brain at 1/250 dilution in carbonate-bicarbonate buffer (50 mM sodium carbonate, 50 mM sodium bicarbonate, pH 9.6). Coated plates were sealed individually and were incubated at 40°C overnight. Next day, plates were washed 5 times with 300 µL of PBS with 0.1% of Tween 20 (PBS-T) and 200 µL blocking buffer (PBS containing 5% skim was added to each well and incubated during 40 min at 37°C. After another five washing in the same conditions 50 µL of birds serum samples at 1/10 dilution was added and incubated 2 h at 37°C. Plates washed again five times and Mabs were diluted in blocking buffer at the specified dilution, added to each well (50 µL), and incubated for 1 h at 37°C. Plates were washed and 50 µL of horseradish peroxidaseconjugated rabbit anti-mouse IgG (Amersham) was added at a dilution of 1:2,000 to each well and again incubated for 1 h at 37°C, followed by five washes more. Equal volumes of OPD (Orthophenilendiamine) and peroxidase solutions were mixed, and 50 µL was added to each well. The optical density at a wavelength of 492 nm was determined with an automated plate reader. The percent inhibition of Mab binding was calculated [8] as 100-[(TS-B)/(CS-B)]-100, where TS is the mean optical density of the test serum, CS is the mean optical density of the control serum (from uninfected chickens), and B is the background optical density.
Mosquitoes were captured in five zones of La Sierpe municipality; P5, El Jibaro, Pista Campos de Arroz, Pista de Aviones and La Sierpe town. They were trapped with CDC light traps (models 512), UV traps and gravid traps in the selected localities. Each site was monitored during a week. Traps were placed in late afternoon and retrieved the next morning.
Entire collection from each trap, was killed by freezing, mosquitoes were transferred to 2 mL polypropylene cryotubes labeled vials. Mosquitoes were pooled according to the species, genera, locality, date, trap number, trap type and number of mosquitoes. Vials were kept on dry ice until they were shipped to the laboratory. Mosquito pools were triturated in 1000 µl de BA-1 medium (1 × 199 medium with Earl’s salts to final volume of 100 ml 0.05 M Tris buffer, pH 7.6, 1% Bovine Serum Albumin, 4.7 ml 7.5% NaHCO3 solution and 100 units/ml of penicillin/streptomycin) and homogenized by using copper beads Premium BBS (Crosman Corporation E Blomfield NY 14443 1-800-7 Airgun) and agitation by vortexing. Aliquots of 200 µL were collected from each grinded sample and submitted to isolations and biomolecular assays.
Virus isolation from mosquito homogenates pools was performed using Aedes albopictus cell line (clone C6/36-HT). Aliquots of 100 µL from mosquito homogenates were added to inoculate C6/36-HT cell monolayers in cell culture tubes. One mL of MEM medium containing 2 mmol/L glutamine, 2% of heat-inactivated Fetal Bovine Serum (FBS) with antibiotics (penicillin and streptomycin) was added after 1 h incubation at 33°C temperature. The culture was monitored daily for detect Cytopathic Effects (CPE). Cell monolayers that showed CPE were harvested subsequently. In order to identify the infectious agent as flavivirus infection, cells from every isolation experiments tube were washed twice in PBS and dried on 10 well microscope slides; afterward they were fixed on acetone for 10 min and store at -70°C until used, IFA screening of the slides was performed using anti-flavivirus 4G2 mAb, RT-PCR.
RNA extraction from homogenates from mosquitoes and from viral isolation in C6/36 HT was done using the Qiagen RNA easy kit with the manufacturer’s recommendations. A Taqman real-time RT-PCR assay was designed following the Standard Operating Procedure: West Nile Virus Mosquito Testing via Real-Time Taqman® RT-PCR, Section: Field Studies, SOP Number: 2003-1 by using generic primers (Table 1).
| Arbovirus group | Primers | Molecular protocols | Region of genome/Size (bp) |
| Flavivirus | WN3’ NC-forward CAGACCACGCTACGGCG WN 3’ NC-reverse CTAGGGCCGCGTGGG WN 3’ NC-probe TCTGCGGAGAGTGCAGTCTGCGAT |
Taqman real-time RT-PCR | 10,668-10,684
10,770-10,756 10,691-10,714/103 |
| Flav1 AATGTACGCTGATGACACAGCTGGCTGGGACAC Flav2 TCCAGACCTTCAGCATGTCTTCTGTTGTCATCCA |
RT-PCR | NS5 9273-9305
10,102-0,136/863 |
|
| CFD2 GTGTCCCAGCCGGTGTCATCAGC MAMD AACATGATGGGRAARAGRGARAA |
RT-PCR | NS59232-9258 9006-9029/252 |
Table 1: Primers and molecular protocols for flavivirus detection in target groups
Real-time RT-PCR of mosquito isolations was performed by using RNA extracted by RNeasy 96 Viral Isolation Kit (Qiagen, catalogue#204443). Briefly, Real-time RT-PCR were done as follow, reverse transcription was at 50°C for 20 minutes, followed by Hot Star Taq activation at 95°C for 15 minutes, 2 steps cycles, denaturation at 95°C and 60°C for 1 minute, 20 seconds for annealing and extension. A cycle threshold value<38 was used to identify and determine Taqmanpositive simples.
Also, a conventional RT-PCR with generic flavivirus primers (Table 1) was performed from mosquitoes’ homogenates and its isolation in C6/36 HT. Briefly, 10 µl of RNA was added to a 50 µl final volume containing 5 µl One Step RT-PCR buffers 5x, 1 µl dNTPs, 5 µl Q solution, 1 µl PCR primers and 2 µl of One Step RT-PCR Enzyme Mix. RT (at 50°C for 30 min) was followed by a denaturation step at 95°C for 15 min and 45 cycles of amplification (94°C for 30 s, 60°C for 1 min, and 72°C for 1 min) with a final extension step at 72°C for 10 min.
A total of 351 adult birds were bled. The birds belonged to 32 species (Table 2) from them 284 were terrestrial and 67 aquatic (Tables 3 and 4). The 17% of terrestrial birds had antibodies to flavivirus and the 1.4% to WNV. House sparrow, a permanent and non-migratory species, was the specie with the highest percentage with antibodies to flavivirus (10.2%) and WNV (1%). The 56.7% of aquatic birds exhibited antibodies to flavivirus and the 11.9% to WNV. In aquatic birds 61% from the total showed antibodies to flavivirus meanwhile 13.4% were positive to WNV antibodies.
| Scientific name | Common name | Scientific name | Common name |
| Dumetellacarolinensis | Gray Catbird | Seiurus aurocapilla | Ovenbird |
| Mimus polyglottos | Northern Nockingbird | Parkesia noveboracensis | Northern Waterthrush |
| Parula americana | Northern Parula | Parkesia motacilla | Louisiana Waterthrush |
| Setophaga magnolia | Magnolia Warbler | Geothlypis trichas | Common Yellowthroat |
| Setophagatigrina | Cape may Warbler | Passerdomesticus | House Sparrow |
| Setophaga caerulescens | Black-throated Blue Warbler | Divesatroviolaceus* | Cuban Blackbird |
| Setophaga coronate | Yellow Rumped Warbler | Molothrus bonariensis | Shiny Cowbird |
| Setophaga discolor | Praire Warbler | Tiaris canorus* | Cuban Grassquit |
| Setophaga palmarum | Palm Warbler | Tiaris olivaceus | Yellow-faced Grassquit |
| Mniotiltavaria | Black and White Warbler | Zenaida macroura | Mourning Dove |
| Setophagaruticilla | American Redstart | Columbina passerina | Common Ground Dove |
| Helmitheros vermivorum | Worm-eating Warbler | Charadriusvociferus | Killdeer |
| Anas discors | Blue-winged Teal | Coccyzus merlini | Great lizard-cuckoo |
| Crotophaga ani | Smooth-billed Ani | Himantopus mexicanus | Black-necked Stilt |
| Plegadis falcinellus | Glossy Ibis | Tringa melanoleuca | Greater Yellowlegs |
| Tringaflavipes | Lesser Yellowlegs | Calidris minutilla | Least Sandpiper |
Table 2: Species of birds captured
| Species | Antibodies to Flavivirus | Antibodies to WNV | Residence status |
| Gray Catbird | 1 (0.35%) | 1 (0.35%) | Migratory winter resident |
| Northern Mockingbird |
1 (0.35%) | - | Permanent resident non migratory |
| House Sparrow | 29 (10.2%) | 3 (1.05%) | Permanent resident non migratory |
| Cuban Blackbird* | 1 (0.35%) | - | Permanent resident non migratory |
| Shiny Cowbird | 2 (0.7%) | - | Permanent resident non migratory |
| Yellow-faced Grassquit | 7 (2.46%) | - | Permanent resident non migratory |
| Great lizard- cuckoo | 1 | - | Permanent resident non migratory |
| Smooth-billed Ani | 4 | - | Permanent resident non migratory |
| Cuban Grassquit* | 4 | - | Permanent resident |
Table 3: Terrestrial birds with antibodies to flavivirus and WNV
WNV: West Nile Virus;
* Endemics species
| Species | Birds with antibodies to Flavivirus | Birds with antibodies to WNV | Residence status |
| Blue-winged Teal | 10 (15%) | 3 (4.47%) | Winter resident |
| Black-necked Stilt | 10 (15%) | - | Bimodal |
| Greater Yellowlegs | 1 (1.49%) | - | Winter resident |
| Glossy Ibis | 13 (19.4%) | 5 (7.46%) | Bimodal |
| Lesser Yellowlegs | 1 (1.49%) | - | Winter resident |
| Least Sandpiper | 3 (4.47%) | - | Winter resident |
Table 4: Aquatic birds with antibodies to flavivirus and WNV
Three hundred and four human samples were taken in La Sierpe (137 samples) and El Jíbaro (167 samples) localities. From La Sierpe samples 29.1% were positives to dengue IgG antibodies and 47.5% had neutralizing to dengue 2. Just two samples from each locality showed WNV IgG antibodies by ELISA Focus Tech. and their presented specific neutralizing antibodies with titers of 1:80 by PRNT. In El Jíbaro locality, the 28.7% of samples were positives to dengue IgG antibodies and 20.8% of them showed neutralizing antibodies to Den 2. Samples from both localities were negative by PRNT to SLEV.
Two hundred thirty four pools of 9 species of mosquitoes were collected (An. albimanus, An. crucians, Oc. sollicitans, Oc. scapularis, M. titillans, Cx. nigripalpus, Cx quinquefasciatus, Ps. sp. y Ps. confinnis). The Cx. quinquefasciatus was the most representative species with the highest prevalence of infection in both localities studied (Table 5).
| Location | Prevalence of mosquitoes (No positive pools/no total pooled tested RT-PCR/ primers Flav1/2) |
Prevalence of mosquitoes (No positive pools/no total pooled tested. RT-PCR/ primers MAMD/CFD2) |
| La Sierpe | 15.7 % (3/19) | 10.5 % (2/19) |
| El Jibaro | 0% (0/19) | 21 % (4/19) |
Table 5: Frequency of flavivirus RNA positives samples from homogenate Culex quinquefasciatus pools
Sixty six viral isolations from Cx. quinquefasciatus, Cx. nigripalpus, An. albimanus, Oc. solicitans and M. titillans were achieved and all of them showed citopatic effect and were positive to flavivirus by IFA. The RNAs extracted from them and assayed by Real-time RT-PCR for WNV detection were negative in all cases. However, the existence of CPE and the positivity by IFA suggested the presence of flavivirus into C6/36 primers. This result lead to perform a conventional RT-PCR with flavivirus specific primers (Flav 1 Flav 2 and CFD2 and MAMD primers) for the NS5 coding region of the RNA [13-15].
RNAs from mosquitoes homogenates and isolations in C6/36 were used with 2 set of primers. All samples from isolations and just 3 samples from homogenates were positive for Flav-1 and Flav-2 primers and presented a band equivalent with to NS5 with a molecular weight of 863 bp corresponding to Flavivirus genera (Figures 2A-2D). On the other hand 7 samples come from homogenates and 8 samples from isolations were positive with CFD2 and MAMD primers (Figures 3 and 4).
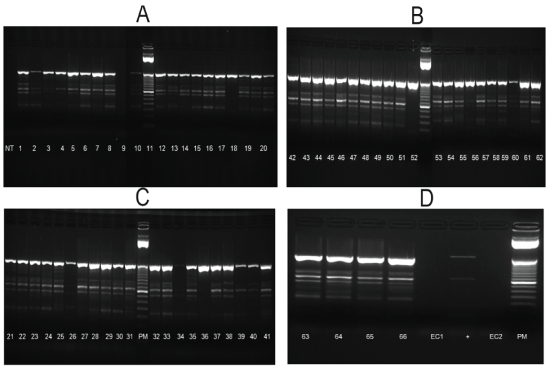
Figure 2: One-Step RT-PCR on C6/36 Isolates using Generic Flavivirus Primers Flavi 1 and Flavi 2.
MW: Molecular Weight; NC: Negative Control; RNA extraction from C6/36 cells; PC: Positive Control; WNV RNA
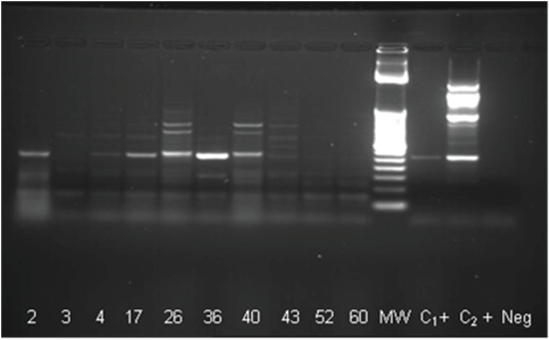
Figure 3: One-Step RT-PCR with MAMD/CFD2 primers on mosquito’s homogenates
MW: Molecular Weight; NC: Negative Control (not template); C+: Positive control
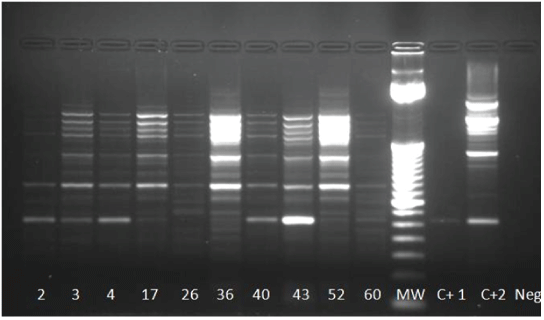
Figure 4: One-Step RT-PCR on isolates using CFD2 and MAMD primers. Positives samples 2, 3, 4, 17 (weakly), 36, 40, 43 and 60; MW 252 pb
MW: Molecular Weight; NC: Negative Control RNA extraction from C6/36 cells; PC: Positive Control; WNV RNA
WNV is maintained in nature as other flavivirus like SLEV, Western Equine encephalomyelitis, and Eastern Equine encephalomyelitis in a cycle between birds and mosquito species mainly Culex mosquitoes [16]. Migratory birds are supposed to be the most important amplifying hosts of WNV contributing to spread this virus and also SLEV in urban locations in North America [17,18].
Flavivirus circulation in Cuba has been reported by the presence of antibodies in animals and humans. Human antibodies to flavivirus have been stated from national serological surveys [19] or during flavivirus surveillance [8] and this allowed to know the circulation of some of them as Eastern Equine Encephalitis Virus (EEEV), dengue, SLEV, WNV, Chikungunya (CHIK) and Zika [20]. Serological studies have demonstrated of WNV circulation in Cuba and its permanence in humans up to 2011 [9,21].
As these results point out, the total prevalence of dengue human antibodies was higher (57.8%) in La Sierpe municipality, than the rest of flavivirus assayed (0.65% to WNV and 0% to SLEV). The low incidence of WNV illness in Latin American countries has been argued since a lot of points of view. Among them, the fact of preexistence of heterotypic flavivirus antibodies that can influence in a population previously infected with DENV making them resistant or less susceptible to WNV [6,22]. In our case, this assertion could be an explanation since several dengue outbreaks and epidemics have been report since when dengue laboratory surveillance was established in Cuba [20].
Previous studies in birds, have demonstrated the presence of antibodies to flavivirus specifically SLEV and EEE virus during 1988, 1989 and 1994 [23] and for WNV in Gallus gallus domesticus in 2011 [24].
After the expansion of WNV in United States, Cuban surveillance in birds was established and it concluded in 2005 after the appearance of the first WMV human cases. During these years, significant bird mortality was not observed. A total of 1828 dead birds, received from national passive surveillance, were studied by PCR being all of them negative. The birds sampled belonged mainly to Caradriiformes, Passeriformes, Anseriformes, Columbiformes, Gruiformes, Strigiformes, Pelecaniformes, and Ciconiiformes orders with more than 200 species among them (source National Surveillance Laboratory, Tropical Medicine Institute, Havana Cuba).
The introduction of WNV in Cuba is unknown [8]. Given the speculation that WNV may be spread by migrating birds, and later the demonstration that migrating passerine birds are potential dispersal vehicles for WNV [25] it is assumed, taking into account our results and the birds species involved, that the virus was introduced in the country by this way
Most species that showed flavivirus antibodies have been reported as permanent or winter residents in cays of the Jardines de la Reina archipelago, close to S. Spiritus province in the south of Cuban Island [26]. Some of them like Northern Mocking bird have previously been reported with flavivirus antibodies including WNV [27-29]. In relation to the species with WNV specific antibodies, 2 were aquatic and terrestrial migratory winter resident (Blue-winged Teal and Gray Catbird) and 2 permanent residents (House Sparrow, Glossy Ibis).
Sancti Spíritus province, specifically Sur del Jibaro rice paddies in La Sierpe municipality has been one of rice-growing regions studied in relation to the importance of rice paddies to Cuban birds [30- 32]. These researches have revealed that most birds recorded are totally or partially coming from North America and are represented by the orders Podicipediformes, Pelecaniformes, Ciconiiformes, Anseriformes, Gruiformes and Charadriiformes [32] in this orders six species of our research are included.
Gray Catbird is one of the frequent migratory species wintering in Cuba as Blue-winged Teal [33]. Gray Catbird has been deeply studied in its relation to arboviruses, demonstrating its competence as reservoir for EEV and its significant role in maintenance and spread of WNV [34].
Blue-winged Teal is the migrant waterfowl most frequently recorded and abundant in Cuba where has a great influence on the control of various unpleasant weeds in the rice fields [35]. Until now, we have not get records of WNV in Blue-winged Teal but its role as possible reservoir of avian influenza virus together with other species of the Anatidae family had been suggested [35,36]. It is known that Bluewinged Teal performs a long-distance migration to Central America, the Caribbean, and some areas of South America. It is one of the first species to migrate south and one of the last to return to the north [36,37] and these characteristics can contribute with the geographic spread and keep them as available reservoirs for virus infection at the migration sites.
Glossy Ibis bimodal species has deeply studied among water birds in rice fields in Cuba; mainly in the rice field complex of Sur del Jibaro, and it has been associated with eastern equine encephalitis (EEE) virus [38] with high tittered viremia and high mortality rate [39]. In relation to WNV, in this bird species neutralizing antibodies have been found in UK and Spain although at low titers [40].
House Sparrow was the species with more number of birds with antibodies to WNV. This behavior can be possible due to its cosmopolitan behavior so it can be found in urban, suburban, and rural landscapes [41] which have supported its implication in the transmission cycles of SLEV and WEEV. Related to WNV, field studies during and after WNV outbreaks have confirmed House Sparrows as important amplifying hosts. Also taking into account the viremia values, is considered highly competent for infecting mosquitoes with WNV as most passerine birds [42-44], who also are blood-meal sources to feed exclusively Culex mosquito species [45]. In our study, this species was the most collected (91%) and Cx. quimquefasciatus was the predominant.
All mosquitoes pools were positive to flavivirus presence by RTPCR. Previous entomological and ecological studies in S. Spiritus province have demonstrated that Cx. quinquefasciatus was the species with more frequency and dispersion in the region [46,47]. Genera of Anopheles and Ochlerotatus also had high frequency and relative abundance [47]. Studies on this mosquito species have indicated its preference, mainly to birds from Passeriformes, including House Sparrow, House Finch, Gray Catbird, and American Robin [48,49] and this behavior has been found in different regions like US, Brazil, Australia and Hawaii [50] thus on the basis of this characteristic Cx. quinquefasciatus makes possible the transmission of WNV to incidental hosts, including equines and humans.
Despite we had almost all the factors (previous cases of WNV in the region, culicid fauna able to circulate WNV and susceptible hosts who act as primary or amplifiers hosts to the virus) to “catch” WNV this did not occurred. Real Time PCR for WNV was negative for all isolations.
The RT-PCR with flavivirus specific primers (Flav 1 Flav 2 and CFD 2 and MAMD) demonstrated bands with molecular weights of 832 bp and 252 bp respectively, corresponding to Flavivirus genera. The use of samples from viral isolations in C6/36 and from homogenates of mosquitoes, suggest the presence of viruses of this genus and reject the possibility of C6/36 HT cells of any ISVs contamination.
The ecology of ISVs and WNV is totally unknown in Cuba. These results represent the first step to fill the gap about flavivirus ecology in the Island. An enormous door is opened to answer a lot of questions: are Cuban birds resistant to WNV? It is an avirulent strain which circulate in the country? Which species of Cuban mosquitoes are more competent for flavivirus transmission? Is an ISVs circulating in a variety of mosquitoes species? Is this potential infection a barrier to other flavivirus circulation? Further researches to identify this possible ISV and to answer all these questions are ongoing.
The opinions expressed by authors contributing to this journal do not necessarily reflect the opinions of the Institute of Tropical Medicine “Pedro Kouri” or the institutions with which the authors are affiliated.
Firstly, we would like to thank to Michael Drebot, Robbin Lindsay, Harvey Artsob and Antonia Di Bernardo and the team from Zoonotic Diseases and Special Pathogens, National Microbiology Laboratory, Public Health Agency of Canada for all the support. Also to ornithologists of Institute of Ecology and Systematic, Presidency of Provincial Government, Provincial Direction of Health, Provincial Direction of Veterinary, Provincial Direction of Veterinary Services of Frontiers, Provincial Direction of Flora and Fauna, Provincial Direction of Border Guards, Provincial Direction of Civil Defense, Fishing Company, Department of Epidemiology, Provincial Direction of Control of Vectors, Sancti Spiritus province and Institute of Tropical Medicine Pedro Kourí. We are deeply thankful to Lourdes Mugica and Martin Acosta for their critical review and suggestions.
Download Provisional PDF Here
Aritcle Type: RESEARCH ARTICLE
Citation: Pupo-Antúnez M, Vázquez Y, Andonova M, Vázquez S, Morier L, et al. (2018) Field Study: Searching for West Nile Virus in Cuba. J Emerg Dis Virol 4(1): dx.doi.org/10.16966/2473-1846.142
Copyright: © 2018 Pupo-Antúnez M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Publication history:
All Sci Forschen Journals are Open Access