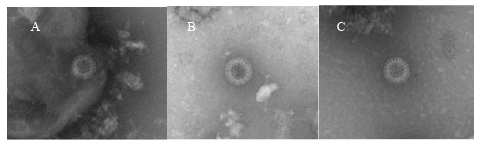
Figure 1: Electron micrographs of GCRV particles in rectal swab samples of children with diarrhea. Panels A, B and C: complete GCRV particle in samples from patients 1, 3 and 6. Bar=100 nm
Ken Sugata1,5* Kimberly Foytich1 Sung-Sil Moon1 Maureen Metcalfe2 Tetsushi Yoshikawa3 Naoko Nishimura4 Takao Ozaki4 Baoming Jiang1
1Division of Viral Diseases, Centers for Disease Control and Prevention, Atlanta, GA, USA*Corresponding author: Ken Sugata, Department of Pediatrics, Fujita Health University, the Second Teaching Hospital, Otobashi Nakagawaku, Nagoya-shi, Aichi, Japan, E-mail: ksugata@fujita-hu.ac.jp
Background: Group C rotavirus (GCRV) has been detected in both sporadic cases and outbreaks of gastroenteritis (GE) throughout the world. However, the epidemiology of GCRV infection is still poorly understood because detection methods are not readily available compared to group A rotavirus (GARV).
Objective: We assessed molecular and enzyme-linked immune assays for the diagnosis of sporadic GCRV infection and investigated clinical symptoms including extraintestinal findings.
Study design: Thirty hospitalized children with acute GE were enrolled for the diagnosis of GARV and GCRV, and the comparison of clinical symptoms between the two groups.
Results: Of 30 rectal swab specimens tested, 24 were positive for rotavirus; 6 were positive for GCRV by enzyme immunoassay, RT-PCR and electron microscopy, whereas 16 were positive for GARV. Although Vesikari severity scores for GE were not significantly different between GCRV and GARV patients, extraintestinal complications such as seizures and hepatitis were not seen in GCRV patients.
Conclusion: GCRV sporadic infection was seen at the same time of GARV prevalence in the same area. A combination of different assays is needed for more accurate diagnosis of GCRV infection.
Rotavirus; Gastroenteritis; Transaminase levels; Polyacrylamide gel electrophoresis
ELISA: Enzyme Linked Immunosorbent Assay; GARV: Group A Rotavirus; GCRV: Group C Rotavirus; PCR: Polymerase Chain Reaction.
Rotavirus (RV) is the major cause of gastroenteritis (GE) in young children worldwide. RVs have been subdivided into seven groups (A to H) on the basis of their dsRNA electrophero types and antigenicity [1]. In humans, Group A rotavirus (GARV) was first identified in 1973 [2]. GARVs have been associated with high morbidity and mortality with an estimated 215000 deaths occurring annually [3], causing infants severe dehydration because of fever and frequently diarrhoea and vomiting. Furthermore it has been widely known that GARV GE is sometimes complicated by high fever, elevated transaminase levels [4,5], seizures [6,7] and encephalopathy [8,9] which may be caused by systemic viral infection. In Japan, the most common viruses responsible for acute GE are norovirus, GARV and sapovirus [10]. Group C rotavirus (GCRV) was first identified in swine in 1980 [11]. Human GCRV infection was first detected in Rio de Janeiro, Brazil in 1983 [12]. Subsequently, GCRV has been detected in both sporadic cases and outbreaks of GE throughout the world. The detection rate of GCRV infection has been reported, ranging from 0.23% to 6.9% in many countries [13-17]. In Japan, GCRV was first reported in 1986 [18] and the positive rates in sporadic cases and outbreaks ranged from 2.7-13.3% [19]. GARV antigen detection using commercial ELISA kit is fast, easy and handy; however, an ELISA kit for the detection of GCRV is not still available. Many assays and molecular techniques such as electron microscopy, ELISA, reverse transcriptionpolymerase chain reaction (RT-PCR), polyacrylamide gel electrophoresis (PAGE) and reverse passive hemagglutination (RPHA), have been able to detect GCRV in several studies. There is not gold standard for GCRV detection, then due to the lack of sensitive and specific diagnostic assays, the epidemiology and disease burden of GCRV have not been fully established. In addition, little is known about GCRV clinical symptoms in humans; GCRV is generally believed to be associated with milder GE or asymptomatic infection in children and adults [20]. Therefore, it is very important to accumulate samples and information, even from sporadic cases. In the present study, we assessed molecular and enzymelinked immune assays for the diagnosis of sporadic GCRV infection in a small group of non-immuno compromised children in Japan. We further compared clinical symptoms of GE between GARV and GCRV infection in these children.
Thirty children (17 boys and 13 girls) under 10 years old, hospitalized at the pediatric department in Konan Kosei Hospital between March and June of 2009 with acute GE, were enrolled in this study. At the time of admission, a rectal swab sample was collected from each patient. In addition, pair of serum samples for RV antigen testing were collected both at the time of admission (Day 1) and discharge from Day 3 to Day 8, several days later. Samples were stored at -70°C before being shipped on dry ice to the Centers for Disease Control and Prevention (CDC) in Atlanta, USA. Clinical manifestations of the subjects were ascertained retrospectively from medical records. We assessed the severity of disease by using a 20-point Vesikari scoring system [21]. Written informed consent was obtained from all parents and the children were then enrolled using the study protocol approved by the institutional review boards of the Fujita Health University (08-177). The CDC tested coded specimens with unique identifiers for anonymity under a non-disclosure agreement, which unequivocally prohibits the release of personally identifiable information about participants.
In addition, rectal swab samples from all patients were cultured to detect enteric bacteria.
All 30 rectal swab samples were screened for GARV and adenovirus by using commercial ELISA kits (Rotaclone and Adenoclone; both from Meridian Bioscience, Inc., Cincinnati, OH). All stool specimens were tested for GCRV antigen using an in-house immunoassay with reagents specific to the porcine GCRV Cowden strain [13]. Briefly, 96-well plates (Nalgene Nunc International) were coated with porcine anti-GCRV serum (U340; dilution 1:2000) or normal porcine serum (Z1329; dilution 1:2000) in coating buffer (35 mM NaHCO3 , 15 mM Na2 CO3 , pH 9.6). After washing with phosphate buffered saline (PBS)-0.05% Tween (PBST) plates were incubated with blotto. Plates were incubated with diluted stool samples (dilution 1:10) or GCRV virus-like particles (VLPs, positive control). Plates were incubated with rabbit hyper immune serum to human GCRV VLPs (CD94; dilution 1:4000) Plates were incubated with horseradish peroxidase-goat anti-rabbit IgG (KPL, Gaithersburg, MD, USA) diluted 1:5000, for 1 hour at 37ºC. Reactions were stopped with 1 N HCl and the optical density (OD) was read at 450 nm with an ELISA reader (MRX Revelation, Dynex Technologies, Chantilly, VA). A sample was considered positive if the ratio of absorbance with the hyperimmune serum (U340) over the normal serum (Z1329) was >1.7 [13].
All rectal swab specimens were tested for GCRV by RT-PCR and nested PCR with previously published specific primers for the VP6 and VP7 genes of human GCRV (17, 22). VP6 and VP7 genes were amplified using primers BMJ44 and BMJ145, and BMJ13 and BMJ107, respectively. Nested PCR was done for VP6 and VP7 using primers BMJ43 and BMJ144, BMJ27 and BMJ143, respectively. The nested PCR was performed as RTPCR without reverse transcription and with annealing temperatures of 53ºC and 48ºC for VP6 and VP7 genes, respectively. The PCR products of GCRV VP6 and VP7 genes were purified by mini columns (QIAquick, Qiagen, Valencia, CA) and sequenced using the ABI-PRISM Big Dye terminator Cycle Sequencing kit and an ABI prism 310 Genetic analyzer (Applied Biosystems Inc. Foster City, CA).
G genotypes of GARV in rectal swab sample were determined by using RT-PCR with previously published specific primers 9con1L and VP7- RDg [23]. The second amplification was performed from the first PCR product (1025 bp) using the 9con1L primer and a cocktail of G typespecific primers (9T-1, 9T-2, 9T-3P, 9T-4 and 9T-B) for VP7 G1-G4 and G9, respectively. The amplicon sizes for G1, G2, G3, G4 and G9 strains were 158 bp, 224 bp, 466 bp, 403 bp and 110 bp, respectively. VP4 P typing was performed in the same manner as VP7 gene assay with previously published specific primers Con2 and Con3 [23]. The second amplification was performed from the first PCR product (877 bp) using the Con3 primer and a cocktail of P type-specific primers (2T-1, 3T-1 and 1T-1) for P4, P6 and P8, respectively [24]. The amplicon sizes for P4, P6 and P8 strains were 484 bp, 268 bp, 346 bp, respectively.
The rectal swabs in 10% PBS were examined by negative stain electron microscopy using two different techniques. First, 2 l of 1:10 diluted stool suspension was pipetted onto a 1% alcian blue treated formvar-carbon coated 300 mesh nickel grid (EMS Cat. No. FCF300-Ni). Samples were incubated overnight on grids in a refrigerator at 4ºC. Sample were then blotted, rinsed with 3.5 µl of 1% bacitracin [25], blotted and stained with 3.5 µl of 5% ammonium molybdate (pH 6.9) with 1% trehalose (w/v) [26]. The stain was immediately blotted after applying and the grid was then allowed to air dry prior to viewing at the transmission electron microscope (120 kV, BioTwin, FEI, Hillsboro, OR). If virus was not observed utilizing the first method, a second method was used to concentrate the samples onto the grids. Again, a 1% alcian blue treated grid was placed in an adapter located in an Ultra-Clear™ Beckman Airfuge® polyethylene terephthalate micro centrifuge tube (Beckman Coulter, Inc., Fullerton, CA Cat. No.345843; 5 mm × 20 mm). The grid and adapter were submerged in 80-90 µl of 1:10 diluted stool suspension. The samples were then ultracentrifuged at 20-24 pounds per square inch using a Beckman Airfuge® (Beckman Coulter, Inc., Fullerton, CA). After 10 minutes, the grids were blotted and stained as described above.
A sample that tested positive by more than two independent diagnostic assays was considered positive for GCRV.
Statistical analyses among clinical variables were performed using SPSS version 21 (IBM, Armonk, NY). Mann-Whitney U test procedure was employed to analyze mean variables such as age, duration of stay, Vesikari severity score (0-20 points; severe >11 points), and duration of individual clinical symptoms (e.g., fever and vomiting) between GCRV and GARV groups. Gender and presence of hepatitis and convulsion in patients with GCRV and GARV GE were compared using Fisher’s exact probability test. In all comparisons, a p-value < 0.05 (two-tailed) was considered statistically significant.
In this study, hepatitis was defined as elevated transaminase levels (alanine amino transferase: 50 IU/L). Instead, convulsion was defined as a cerebral paroxysm without signs of central nervous system infection, which has violent muscle contractions and loss of alertness with or without fever during GE.
All 30 patients were generally healthy before developing GE. The mean duration for each patient’s hospital stay was 7.1 ± 1.6 days. The patients had a mean Vesikari score of 12.9 ± 2.4 points; 25 had a high severity of GE. Of the 30 rectal swab samples tested by ELISA, 16 were positive for GARV. Fourteen of the 16 ELISA-positive samples were G and P typed by RT-PCR; Eight patients were infected with G1P, two with G9P, two withG3P, and one with mixed G1/3P [8]. One sample was G and P non typeable.
We detected GCRV antigen in 9f the 30 rectal swab samples by ELISA. ELISA-positive specimens were then tested by RT-PCR using primers specific to GCRV VP6 and VP7 genes and 5 specimens were positive (Table 1). We further examined ELISA-positive samples by EM. This positivity was confirmed by EM in three PCR positive samples containing RV or RV-like particles; 2 PCR positive samples had no detectable particles by EM vice versa1 PCR negative samples resulted positive by EM (Figure 1). In general, GCRV particles were sparse and of poor structural integrity when compared with those of GARV. Thus, of 30 patients examined, 16 were positive for GARV, 6 positive for GCRV, respectively and 8 were negative for both GARV and GCRV. In 8 rotavirus-negative samples, 2 were positive for salmonella, 1 was positive for campylobacter, 1 for adenovirus, and 4 had no pathogens detected. There was no mixed infection with GARV and GCRV.

Figure 1: Electron micrographs of GCRV particles in rectal swab samples of children with diarrhea. Panels A, B and C: complete GCRV particle in samples from patients 1, 3 and 6. Bar=100 nm
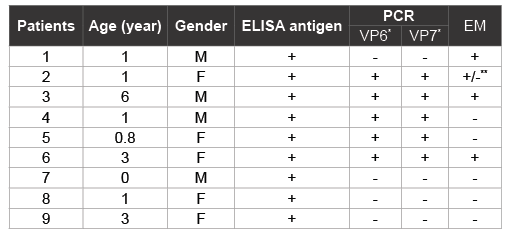
Table 1: Detection of GCRV in fecal samples of children with diarrhea
*
PCR products were confirmed by DNA sequence anaalysis
**Samples contained structures that were similar in morphology to RV cores
We next compared clinical symptoms, including extra intestinal symptoms, between patients infected with GARV and GCRV (Table 2). There was no significant difference in the mean age of the patients between the two groups (1.2 ± 0.5 years in GARV group, 2.0 ± 2.2 years in GCRV group; P=0.747). Though more boys than girls were apparently infected with GARV, there was no significant difference in gender between GARVand GCRV-infected patients (P=0.334). In terms of clinical symptoms, there was no significant difference in duration of hospital stay, Vesikari severity score, and GE symptoms (vomiting, diarrhoea and fever) between the two groups. Although GARV patients appeared to have significantly longer duration of diarrhoea rather than GCRV patients (P=0.021). Of note, GARV patients appeared to be more likely to develop hepatitis than GCRV patients (P=0.046). Only patients with GARV infection had documented convulsions, no patients with GCRV infection had any convulsions. All patients with documented convulsions recovered from acute GE and were discharged without any sequelae.
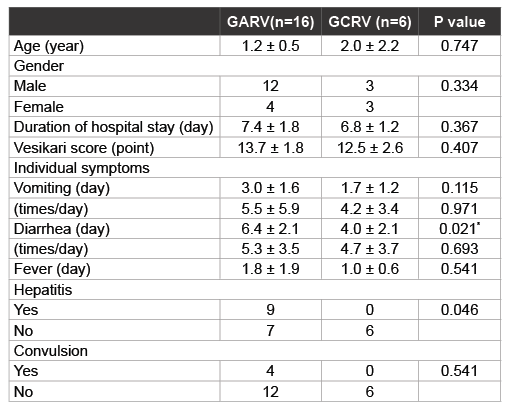
Table 2: Comparison of demographic data and clinical symptoms in children with GARV and GCRV infection
In the present study, 6 of 30 patients were confirmed GCRVs positive by more than two independent diagnostic assays. Although EM might not be specific diagnostic assay to distinguish GARV and GCRV, it is still helpful to visually confirm RV and RV-like particles, which contained structures similar in morphology to RV cores. Supporting our results a previous report indicates that less than 107 virus particles per milliliter in the original specimen would not likely be detected by EM [27]. RV or RVlike particles were identified by EM in 3 of the 6 rectal swabs that tested positive for GCRV by ELISA or PCR. In the case of patient 1, although both VP6 and VP7 RNA were not detected by PCR, RV particles were observed by EM and GCRV antigen was detected by ELISA. Patients 4 and 5 tested positive for GCRV by ELISA and PCR but negative by EM. These findings agree with previous reports showing that GCRVs are fragile and are often present in empty- or core-like particles [28,29], possibly due to their structural instability in the gut or damage during storage and shipment. These data suggest that rectal swab samples could be used to identify RV when it is difficult to collect stool samples from GE patients.
Moreover, our study suggested that ELISA had almost the same sensitivity of RT-PCR but it was less specific than RT-PCR for detecting GCRV in rectal swabs. This indicates that ELISA is an appropriate and useful tool to screen GCRV. However formation of empty capsids or structural instability of the particle reduce the detection rate of RNA by RT-PCR or PAGE, a relatively larger amount of viral proteins than genome may be available for the detection as previously reported [28-34]. That explains why more than two methods could be helpful to increase detection rate of GCRV.
The observations and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Download Provisional PDF Here
Article Type: Research Article
Citation: Sugata K, Foytich K, Moon S-S, Metcalfe M, Yoshikawa T, et al. (2016) Comparison of Clinical Symptoms between Group A and Group C Rotavirus Infections in Japan. J Emerg Dis Virol 2(4): doi http:// dx.doi.org/10.16966/2473-1846.122
Copyright: © 2016 Sugata K, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Publication history:
All Sci Forschen Journals are Open Access