Table 1: Differences between the immune system of a neonate compared to that of an adult
Michaela L Barker1 Andrew W Taylor-Robinson1,*2
1School of Molecular & Cellular Biology, University of Leeds, UK*Corresponding author: Andrew W Taylor-Robinson, Infectious Diseases Research Group, School of Medical & Applied Sciences, Central Queensland University, Rockhampton, QLD 4702, Australia, Tel: +61 7 4923 2008; E-mail: a.taylor-robinson@cqu.edu.au
In sub-Saharan Africa where Plasmodium falciparum malaria is endemic, 80% of the world’s HIV-infected persons reside, making co-infection with these two major human pathogens a common occurrence. Pregnancy is a condition that produces an immunosuppressed state in the mother, which, in combination with an immune compromising disease such as HIV/AIDS, can lead to an increased risk of contracting infectious diseases such as malaria. Co-infection during pregnancy can lead to a number of poor outcomes for both mother and child. There has been little research into the implications of pregnancy and/or co-infection with regard to treatment of HIV/AIDS or malaria, and as a result the options for efficacious drug therapy during gestation are limited. In light of this, prophylactic measures are being investigated, including a vaccine for pregnant women or those planning a family that aims to reduce or prevent the adverse effects of malaria and/or HIV/AIDS. This review discusses the pressing need for treatment, outlines the incumbent difficulties in production of an effective chemotherapeutic or immunization regimen, and considers strategies for present and prospective vaccine development.
Malaria; Plasmodium falciparum; HIV; AIDS; Co-infection; Pregnancy; Placenta; Chemotherapy; Vaccine
Acquired immunodeficiency syndrome (AIDS), caused by the human immunodeficiency virus (HIV), and malignant malaria, caused by the protozoan parasite Plasmodium falciparum, are two of sub-Saharan Africa’s greatest medical challenges with regard to both morbidity and mortality. Of the 36.9 million people infected with HIV/AIDS worldwide at the end of 2014, the most recent year for which statistics are available, 70% live in sub-Saharan Africa, equating to approximately 25.8 million infected individuals out of a population of 949 million [1-3]. Women account for 59% of HIV-infected people in sub-Saharan Africa, creating the potential for around 15.2 million women to have HIV infection during pregnancy [1]. P. falciparum causes between 149-303 million clinical episodes of malaria per year, of which 88% occur in sub-Saharan Africa [4]. The chances of a woman developing malaria during pregnancy are high, but what are the implications of co-existing HIV/AIDS during this period? This review examines the interactions of these two infections and their implications on pregnancy outcome, and assesses current treatments and future prospects for vaccine therapy to eliminate the potential threats posed by both of the major human diseases.
The ‘immunological paradox of pregnancy’, the relationship between a mother and her semi-allogeneic foetus, remains a mystery since it was first recognized by Medawar in 1953. He proposed that foetal rejection does not occur due to lack of foetal antigen expression, either as a result of an anatomical separation between the mother and foetus or due to maternal lymphocyte suppression [5]. Research has since shown that there is no physical separation between the mother and foetus, rather that intimate contact occurs between various foetal cells and maternal immune cells. However, no antigenic stimulation of maternal lymphocytes occurs since foetal trophoblast cells do not express major histocompatibility complex Ia antigens that are required for allograft rejection in humans [6].
The peripheral immune response differs during pregnancy due to a shift in cytokine production towards a type 2 profile [including interleukin (IL)- 4, -5, -9, -10 and -13] promoting humoral immunity, as type 1 cytokines [e.g. IL-2, tumour necrosis factor (TNF)-β and interferon (IFN)-γ] that are involved in cellular immune responses, cause inhibition of embryonic and foetal development. While the cause is unknown explanations include the direct effect of pregnancy-related hormones, placental interference with cytokine production, and trophoblastic production of predominantly type 2 cytokines [6,7].
Although few studies have examined the role of the innate immune system during pregnancy, it is known that numbers and activity of natural killer cells are decreased [7]. Modified monocyte and granulocyte function has been observed through increased expression of some activationassociated adhesion molecules, indicating that these cells are functional during pregnancy [7]. Dendritic cells, the most potent of the antigenpresenting cells, are known to be involved in regulating cytokine balance during pregnancy but further research is required [7,8].
In summary, the innate immune system is active throughout pregnancy, compensating for the suppressed cellular immune response. During infection, immunological responses to bacterial pathogens remain intact, whereas protection is compromised against viruses and other intracellular pathogens that are more efficiently controlled by the cellular immune response, leading to increased susceptibility to diseases caused by such agents [6,7].
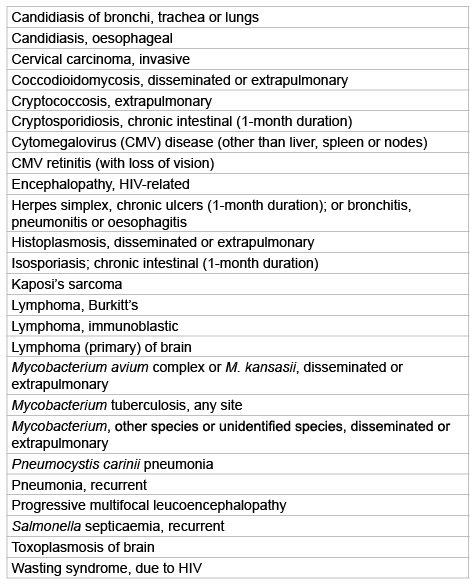
In utero, the foetus is protected by the mother, both physically and by transfer across the placenta of immune factors. The foetus’ own immune system starts to develop in early gestation but by birth it is still incomplete. The level of immune maturity at birth is dependent on to what extent passive immunity has been transferred and also the gestational age of the neonate [8].
Table 1 compares the major components of the immune system of a neonate with that of an adult, illustrating great immaturity in some areas, thereby increasing susceptibility to infection. The placental transfer of maternal immunoglobulin (Ig)G provides extra immunological support at birth, at which time serum levels in the neonate are comparable to those of the mother. The half-life of IgG is 3-4 weeks, and since neonatal levels are negligible at birth and take up to a year to reach adult titers, there is a physiological trough between 3-6 months when there is a deficiency of antibodies as passive maternal IgG levels diminish [8].

Table 1: Differences between the immune system of a neonate compared to that of an adult
Such deficits in neonate immunity may explain the heightened susceptibility to and increased severity of particular infections, such as those caused by Listeria and Toxoplasma [8].
Since the first diagnosis of HIV in 1981 infection has reached pandemic proportions globally, to date close to 78 million people are known to have contracted HIV of whom around half, 39 million, have died from AIDSrelated causes [9]. The virus itself does not kill the human host, but by severely compromising the immune system renders them susceptible to opportunistic infections. Once an HIV patient is infected with an opportunistic pathogen, such as listed in table 2, they are diagnosed as having AIDS [10].
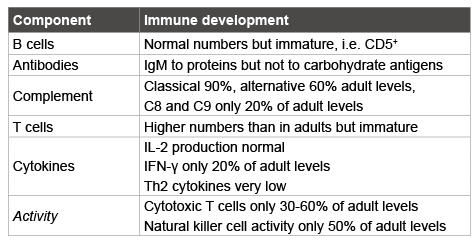
Table 2: AIDS-defining conditions
HIV is a retrovirus characterized by the possession of the enzyme reverse transcriptase that is required for the process of replication of double-stranded DNA from single-stranded RNA [11]. A member of the lentivirus family there are at least two subtypes of virus, HIV-1 and HIV-2. HIV-1 is the more virulent strain and is the cause of most HIV infections worldwide. It is thought to have originated from the common chimpanzee, Pan troglodytes, found in Cameroon [12]. HIV-1 can be subdivided further into group M (major) subtypes, of which there are at least ten, denoted by the letters A-J, and group O (outlier) subtypes, which are found in Cameroon and are highly divergent from group M [11]. HIV- 2 is confined mainly to West Africa and is thought to have originated from the sooty mangabey monkey, Cercocebus atys [13,14]. Both subtypes are spread via the exchange of bodily fluids, mainly through sexual intercourse, intravenous drug use or blood transfusion. Vertical transmission may occur in utero, during vaginal delivery or through breast-feeding [11,14].
The pathogenesis of HIV is based on the intimate relationship formed between the virus and the human immune system. HIV shows tropism principally for the CD4+ cell surface receptor found on T lymphocytes into which it gains entry through an interaction between the CD4+ receptor and its own matrix glycoprotein, gp120, with the aid of the host cell’s chemokine co-receptors CCR5 and CXCR4 [11]. Resistance to HIV infection has been observed in people with mutations in the expression of CCR5 [11]. HIV gains access to CD4+ T cells via dendritic cells, which transport the virus particles to lymph nodes [15].
Incubation of the virus usually takes 2-4 weeks after exposure, during which time the patient is asymptomatic and has negative serology. Seroconversion occurs around 6-8 weeks, sometimes causing nonspecific illness. This is self-limiting and recovery is usually complete within 3 weeks, otherwise the person continues to be asymptomatic. During this acute period, a massive destruction of memory CD4+ T cells initiates immune dysfunction [16]. Clinical latency then occurs, a variable but usually long time during which a rapid viral turnover produces 1010 virions daily. Lymphocytes infected with HIV have a half-life of less than 2 days and as a result 1-2% of the total CD4+ T cell population is destroyed each day. Initially, this is matched by a restorative turnover of CD4+ T cells. However in time CD4+ T cell production fails to compensate for the numbers destroyed, leading to a fall in CD4+ T cell count and an increase in viral load, thus triggering symptomatic HIV infection. In this dysfunctional immunological state the patient may become susceptible to both standard and opportunistic pathogens, prompting the last stage of illness, full-blown AIDS [17].
Highly active anti-retroviral treatment (HAART) has had a positive impact on the lives of HIV patients to whom it is available, prolonging clinical latency by limiting viral replication and thus reducing viral load. This involves the prescription of at least three drugs, usually two nucleoside reverse transcriptase inhibitors (NRTIs) and a protease inhibitor, a nonnucleoside reverse transcriptase inhibitor (NNRTI) or a third NRTI [18]. This combination regimen maximizes the number of active sites available to therapy and limits the risk of development of resistance.
There are concerns that pregnancy may accelerate the progression of HIV/AIDS, either due to suppression of cellular immunity that takes place during pregnancy or due to hormonal changes, but at present there is no conclusive research available [19]. There is more evidence to suggest that HIV adversely affects pregnancy, a systematic review concluding a 1.8- 6 times greater risk of spontaneous abortion when the mother is HIV- 1-positive [20]. Women with HIV infection are also at increased risk of suffering adverse obstetric events such as post-operative endometritis or maternal postpartum haemorrhage [19].
Mother-to-child transmission (MTCT) causes 80-90% of HIV infections in children. This mode of transmission includes in utero, intrapartum and via breast-feeding of the infant. The risk of transmission is multifactorial, depending on the mother’s HIV viral load, mode of delivery of the neonate, and course of treatment where available [21].
Malaria is a parasitic disease caused by Plasmodium species transmitted via the bite of a female Anopheles mosquito. There are five species that infect humans – P. falciparum, P. vivax, P. ovale, P. malariae and P. knowlesi – each distinguished by variations in life cycle stage morphology and sequence and severity of symptoms [22]. Although the global incidence of malaria has fallen in recent years, currently there are still around 300 million episodes of clinical malaria per annum, resulting in an estimated 440,000 fatalities. Sub-Saharan Africa accounts for 90% of deaths, with P. falciparum being the leading cause of mortality [4].
In the mosquito vector the parasite reproduces sexually in the midgut and then asexually in the body cavity, producing sporozoites. These infectious stages migrate to the salivary glands from where they are inoculated into a human host during the next blood feed. Following transfer, sporozoites home to the liver where they reproduce asexually within parenchymal cells forming schizonts containing merozoites, which are released into the peripheral circulation where they rapidly invade erythrocytes. Another asexual reproductive cycle occurs in the blood that leads to erythrocyte rupture and release of new merozoites typically every 46-48 hours for P. falciparum. A small minority of merozoites develop into immature male and female gametocytes, which are the only forms that can infect mosquitoes, taken up at a subsequent blood feed [22,23].
The clinical features of malaria include paroxysms of chills, fever and sweating, and anaemia, the extent of which is dependent on the species of parasite, P. falciparum causing the most severe manifestations [24]. Treatment is complicated by resistance of the parasite to available drugs and of the mosquito to insecticides, both of which are becoming increasingly widespread.
P. falciparum infection poses a significant threat, not only to the mother but also to the foetus and neonate. Maternal mortality rates can be as high as 50% in some areas, which are higher than in non-pregnant women. The risk of malaria causing harm during pregnancy is dependent on the intensity of transmission, level of anti-parasite immunity acquired previously by the mother, and effectiveness of this immunity during pregnancy [25]. Pregnant women are usually most susceptible during the second and third trimester of pregnancy, and are more likely to be symptomatic than non-pregnant women, developing complications such as pulmonary oedema and hypoglycaemia [26]. Placental malaria, another clinical complication that occurs during pregnancy and which has a major impact on neonatal morbidity and mortality, involves inflammation caused by accumulation of asexual erythrocytic parasites in maternal blood spaces of the placenta [26,27].
Under conditions of stable transmission, primigravidae, and to a lesser extent secundigravidae, are most at risk of malaria infection, which is often asymptomatic. Maternal anaemia, which can be life-threatening, and placental parasitaemia are the principal effects of infection on the mother in these areas. The foetus may suffer intrauterine growth retardation (IUGR), leading to low birth weight (LBW) and thus increasing greatly the risk of mortality [28].
All women living in regions of low or unstable malaria transmission predominantly lack acquired immunity to infection and so, regardless of pregnancy history, are equally susceptible to infection. However, the risk of developing severe malaria is 2-3 times higher for pregnant women than for their non-pregnant counterparts. Adverse consequences for the mother remain similar to those in stable areas of transmission, whereas those for the pregnancy may include stillbirth, spontaneous abortion, LBW or congenital infection [29,30].
It is evident that HIV and malaria each has many implications on pregnancy in its own right. Co-infection of these two diseases, without the complication of pregnancy, can lead to a variety of interactions, a few of which have been studied, but the mechanisms remain largely unknown. Several hypotheses have been proposed but the complexity of each disease in itself leads to unpredictable results. For example, the increased risk of acquiring malaria during HIV infection was thought to be as a result of diminished CD4+ T cell numbers, as these cells are responsible for the maintenance of immunity to malaria, but several studies have shown this not to be the case [31,32]. Four hypothetical patterns of interaction may be described:
Acute infection of malaria in HIV-positive individuals results in higher plasma viral loads, regardless of the fact that malaria stimulates production of chemokines, pro-inflammatory cytokines and counterregulatory cytokines, all of which should aid the immune response. The increase in viral load correlates with an increase in the pro-inflammatory response to the malaria parasite, and activation of a systemic immune response initiated by macrophages. However, for unknown reasons in vitro studies have contradicted these findings [32,33].
A summary of 11 studies demonstrates that HIV-infected pregnant women carry a consistently higher risk of malarial parasitaemia, which occurs both throughout pregnancy and at delivery. Parasite densities in these women are also higher in comparison to non-HIV-infected women. This inability to control malaria infection increases a woman’s risk of developing clinical and placental malaria [34].
In the absence of HIV infection women undergoing their first or second pregnancy are at greater risk of malaria infection than are multigravid women. HIV-infected women, irrespective of the number of pregnancies, are all susceptible to the risks associated with malaria [35].
As both HIV and malaria can cause anaemia in pregnancy, a synergistic relationship may occur, demonstrated by the fact that dual infection has been shown to significantly increase the risk of pregnancy-associated anaemia. This is possibly due to an increase in duration of malaria infection during co-infection, as a consequence of higher parasite densities [29].
In HIV-1-positive adults P. falciparum infection can lead to a transient increase in plasma viral load concentrations, but in part this may be reversed by successful anti-malarial treatment. The scale of increase appears to be less in pregnant women compared to adults with symptomatic malaria, although pregnant women are rarely symptomatic. The observed increase is regardless of HIV disease severity and state of CD4+ immunosuppression [36].
Elevated expression of CCR5 on placental macrophages and foetal Hofbauer cells can occur as a result of placental malaria. The availability of CCR5, a major receptor required by HIV-1 virus particles to enter host cells, could lead to a rise in placental viral load and a subsequent increased risk of MTCT of HIV-1 infection. This is consistent with research demonstrating that the majority of HIV-1 virus isolates transmitted from mother to foetus are CCR5-trophic [37].
Women suffering from both HIV and P. falciparum infections during pregnancy have an increased risk of developing clinical malaria, which is defined as documented fever or history of fever in conjunction with microscopically detected parasitaemia. As both diseases separately are known to cause anaemia, with dual infection there is a greater risk of having any anaemia (<11 g/dL) or moderate to severe anaemia (<8 g/dL), possibly due to synergistic activity leading to malaria infection of longer duration and increased parasitaemia [34]. While all pregnant women who are infected with P. falciparum are susceptible to the accompanying obstetrical complications posed by malaria infection alone, such as postpartum haemorrhage and puerperal sepsis, whether or not these risks are greater in dual infection is unknown.
In regard to the neonate, there is an increased risk of a poor birth outcome including LBW (<2500 g), premature birth, and IUGR in dual infection. It may be that LBW reflects the combined effect of shortened gestational age and IUGR. According to data from Malawi, dual infection raises the risk of post-neonatal mortality 3-8 fold compared to HIV infection alone, suggesting an increased risk of MTCT in these infants or an increased rate of progression from HIV to AIDS [38]. More recent evidence shows a decreased risk of post-neonatal mortality in women with co-infection in comparison to HIV infection only, and this protective factor also corresponds with a reduced MTCT risk in co-infected women [34,35].
Interference of transfer of maternal antibodies to the foetus can occur as a result of HIV infection, causing pathological changes to the placenta. This includes antibodies to antigens such as the blood stage peptides merozoite surface protein 3 (MSP-3), apical membrane antigen 1 (AMA-1), variant surface antigens (VSAs), and the pre-erythrocytic stage circumsporozoite protein NANP-5. During a normal pregnancy, Ig transfer is an active process whereby maternal IgG is taken up by FcRN receptors on the syncytiotrophoblast. The mechanism by which this process is disrupted by HIV is currently unknown [34].
Several studies show increased risk of malaria and anaemia in infants born to women infected with malaria during pregnancy, especially during the first 6 months of life. Theories to explain this heightened risk include the possibility of immune sensitization in utero and the development of immunological tolerance. Less research has been performed on the effect of HIV co-infection on susceptibility to malaria in infancy but available information does not show any relationship between the two factors [35]. HIV infection during infancy as a result of MTCT places the baby at risk of severe anaemia [34].
The increased susceptibility of pregnant women to malaria alone is not well understood but it is thought to be related to modifications in systemic and placental immunological parameters. While few studies have considered the impact of HIV in this scenario, current evidence demonstrates the following:
This is an under-researched area, for malaria as a stand-alone disease but also its co-infection with HIV. It is common to find cord blood malarial infection at delivery but clinical infection in the neonate is rarely seen. Placental sequestration may prevent passage of Igs from mother to foetus, which may possibly lead to increased susceptibility of the neonate to diseases such as measles and streptococcal pneumonia [40]. Further investigation is required to establish the effect of co-infection on MTCT of malaria.
Placental malaria has been shown to have a variable effect on MTCT of HIV, and so it may not necessarily be a risk factor. Conflicting results may reflect the complex relationship between maternal immunity to malaria that could either result in stimulation of HIV virus particle replication in the placenta, thereby increasing local viral load, or in potential control of both the severity of malarial infection and HIV replication. A slight imbalance of factors concerning the immune response can lead to either an increased risk of MTCT or a protective effect, as demonstrated in figure 1. There is evidence to suggest that infants born to mothers who at birth have high plasma concentrations of CC-chemokines and strong anti-HIV cytotoxic and suppressor T cell responses have a reduced risk of HIV-1 infection through MTCT [34,39].
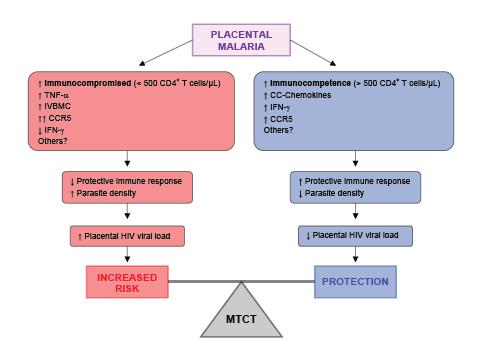
Figure 1: Hypothetical model of immunological modulation of mother-to-child transmission (MTCT) of HIV infection in women co-infected with placental P. falciparum malaria
Transmission of HIV should be preventable by sexual health education of women from an early age. In developing countries increasing access of women to antenatal clinics during pregnancy is an excellent source of contact with patients and provides a good teaching opportunity [40]. In the majority of cases, patients will have already been infected with HIV prior to pregnancy [19], in which case the priorities are to provide HIV testing and counselling, and to prevent MTCT (discussed below).
As mentioned previously, prevention of transmission of HIV to the neonate is achieved, where available, through the prescription of HAART to reduce plasma viral load to <1000 copies/ml. However, in the majority of areas where this treatment is needed, it is unavailable due to lack of resources and funding. If women in resource-poor settings do have access to treatment, this will involve a single 200 mg dose of nevirapine during labor for the mother and one dose of 2 mg/kg of nevirapine for the neonate within 72 hours of delivery. Evidence shows this regimen to reduce the risk of MTCT by approximately 50% [41].
The World Health Organization (WHO) estimates that breast-feeding is responsible for approximately 50% of all infections in children caused by MTCT [29]. In an ideal scenario, resources would be available to enable mothers to provide their child with formula milk and clean water supplies with which to prepare the formula. As this is not the case, many women in resource-poor settings face no alternative but to breast-feed their child. Exclusive breast-feeding, with rapid and early cessation at about 4 months of age, has a decreased risk of MTCT compared to mixed-fed infants [42].
It is recommended by the WHO that all HIV-positive pregnant women receive intermittent preventive treatment of malaria during pregnancy (IPTp), involving a single dose of sulfadoxine-pyrimethamine at least three times during the second and third trimesters of pregnancy, unless the woman is already receiving cotrimoxazole prophylaxis. In non-HIVinfected women, only two doses are required, but there is evidence to show that this is less efficacious in primigravid and secundigravid HIVpositive women. In many parts of Africa, women fear taking sulfadoxinepyrimethamine due to perceived side effects and risk to their unborn child [40,43]. Education and treatment should be provided together as part of routine antenatal care in sub-Saharan Africa and other regions where malaria is endemic. Use of insecticide-treated nets is also recommended by the WHO, which not only protects the mother from malaria during pregnancy, but also the neonate after their birth [44,45].
The generation of a vaccine for HIV has proved difficult due to the diverse genetic variability of the virus, and the fact that the host cells which it targets are essential to contain not only the HIV infection, but also any secondary infections. Any potential vaccine should target induction of both mucosal and systemic immunity in order to combat all modes of transmission. It would also be required to stimulate both innate and cellular immune responses, and to generate high numbers of neutralizing antibody, thereby providing a broad spectrum of protection against all subtypes of HIV [46]. So far, several vaccine concepts, along with a variety of routes of administration and schedules of immunization, have been tested and these can be reviewed through databases created by the International AIDS Vaccine Initiative and the US National Institutes of Health Vaccine Research Center [47]. The concept of using classical vaccination approaches, such as live-attenuated preparations or a wholeinactivated virus, has been shown to have major limitations, so newer strategies have been the focus of ongoing vaccine development [47]. The following information describes current vaccine strategies being researched, some of which are not only being applied to HIV vaccine development, but also to other diseases.
Recombinant envelope subunits: Rather than to introduce the entire HIV virus particle, an envelope subunit can be used to provoke an immune response. In primate models, however, HIV surface glycoproteins such as gp120, gp140 and gp160 stimulate only a transient immune response that does not protect from infection with a virulent strain of simian immunodeficiency virus (SIV) [48].
The Tat protein (trans-activator of transcription) could provide a potential target for vaccine development as it is more conserved than envelope proteins, it is expressed soon after invasion of the host, and it is an essential component of the HIV life cycle. The Tat protein is produced by a small number of RNA transcripts that are created by incomplete transcription of HIV RNA due to a hairpin structure within the RNA. The Tat protein eliminates this structure through binding and phosphorylation of cellular factors. Vaccines based on this protein have demonstrated partially protection and are currently undergoing human clinical trials. It is thought that it may be harnessed both to control virus replication and to prevent onset of disease [49].
Synthetic peptides: This aspect of vaccine development has focused mainly on the V3 loop of the envelope glycoprotein gp120. The immunogen was well tolerated but it failed to invoke the desired response of inducing neutralizing antibodies [50].
Live recombinant vectors: Live attenuated viral or bacterial strains are used as a vector to carry genetic information encoding HIV antigens of interest. These vaccines are able to stimulate both cell-mediated and humoral immunity [48]. Details of vectors currently used in HIV vaccine development are described in table 3.
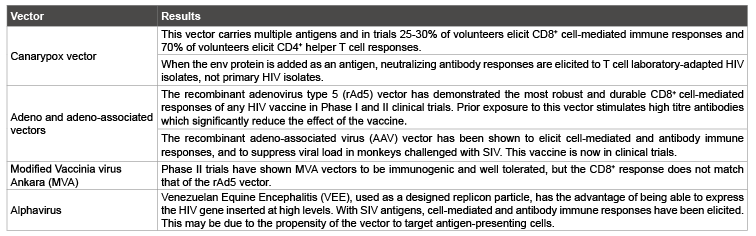
Table 3: Vectors currently used in HIV vaccine development
Naked DNA: Purified plasmid DNA carrying a gene encoding a specific HIV antigen under the control of a mammalian transcription promoter can be administered via either intramuscular or intradermal immunization. This leads to expression of the antigen at the injection site, invoking mainly a Th1-type immune response. As a vaccination method for humans results have been poor, as only weak immune responses are induced compared to those elicited in animal trials [48].
Prime-boost concept: Priming with a nucleic acid vaccine or a viral vector followed by boosting with either another vector or a peptide vaccine has been demonstrated to produce stronger immune responses compared to when either vaccine is given alone. Over past decades many vaccine combinations have been tried, including vaccinia and recombinant envelope subunit, DNA and adenovirus, and adenovirus and canarypox, but so far none has produced satisfactory results in humans.
A current major concern with regard to the design of a vaccine is the hypervariability of HIV, which means that over time antigens may not remain constant and so cannot be included in candidate preparations, and also implies that there is a wide range of variants of HIV, for which it is difficult to provide complete protection with a single vaccine [47]. This diversity is in part a result of being subjected to significant immune pressure imposed by T cell reactivity, enabling HIV to evade given immune responses [51,52]. The potential use of conserved regions as targets for vaccination is also complicated by hypervariability, as HIV may be able to mutate to evade vaccine-induced immunity. Another issue to be resolved is a lack of neutralizing antibody response generated by HIV, through one of its many immune evasion mechanisms, rendering useless a vaccine that would usually take advantage of the production of neutralizing antibodies. Furthermore, there is a need to induce a range of immune responses to adequately prevent disease regardless of whether it is introduced by free virus particles or by virally infected cells. The highly restricted host specificity of HIV means that there is no ideal animal model in which to demonstrate HIV disease and to test candidate vaccine efficacy. Combining these problems with a lack of financial support from the private sector, which is best equipped to develop and manufacture vaccines, and the extended duration of clinical trials required, ensures that production of a successful vaccine will take considerably longer than desired [53]. Current major difficulties encountered on the path towards an efficacious HIV vaccine are outlined in table 4.
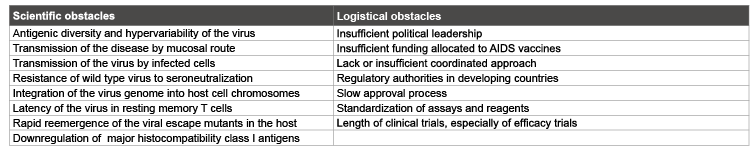
Table 4: Current challenges to HIV vaccine research and development
A slow accumulation of naturally acquired partial immunity to P. falciparum that is observed in residents of endemic areas provides the rationale for development of a malaria vaccine. However, after several decades of research an efficacious vaccine has still to be realized. Progress has been hampered, due partly to the large number of genes (over 5,200) present in the malarial genome that could possibly code for a protective antigen [54]. Many potential vaccine targets within the parasite life cycle are the subject of investigation, as outlined in table 5.

Table 5: Potential targets in the malaria life cycle for vaccine development
The main approach to malaria vaccine design has been to target one of the developmental stages of the parasite; pre-erythrocytic, asexual blood (erythrocytic) and sexual stages [55]. These different targets, along with examples, are described below.
Pre-Erythrocytic vaccines – These aim to prevent establishment of clinical infection by protecting hepatocytes from invasion through production of specific antibodies [56].
Asexual blood stage vaccines – These aims to prevent manifestations of pathology associated with disease, not infection per se, by inhibition of parasite invasion cycles [54].
Transmission-Blocking vaccines – These aims to induce antibodies against sexual stage antigens, preventing the development of infectious sporozoites within mosquitoes [73]. In doing so, communities will be protected from infection, rather than people individually. Vaccines currently undergoing trials contain the P. falciparum ookinete surface antigens Pfs25 and Pfs28, or the P. vivax homologues of these antigens, Pvs25 and Pvs28, and a vaccine based on the latter two antigens has been shown to be safe and to demonstrate modest immunogenicity [73,74].
In regard to pregnancy, the natural build-up of immunity over successive pregnancies due to production of antibodies that recognize placental parasites and which prevent their binding to the placenta suggest the feasibility of developing a pregnancy-associated vaccine [25,75]. Serological studies indicate that a single or finite number of antigens may be required in order to elicit protective immunity based on the production of parasite-specific antibodies [76].
There are major obstacles to vaccine production, including the lack of predictive and reliable animal models, the lack of immunological correlates of protection and the intrinsic antigenic variability of malaria parasites [55]. However, further to sequencing of the P. falciparum genome recent advances in bioinformatics should accelerate vaccine development [77].
The complexity of co-infection of an individual with any two or more diseases has many implications with regard to all aspects of preventive and therapeutic medicine. Pregnancy itself may be described as a selflimiting condition in which immunological effects occur due to the presence of a foreign body. In the scenario of co-infection of HIV and malaria during pregnancy, there are negative impacts on maternal morbidity and mortality, and on the outcome of the pregnancy, but very little is known about how or why this occurs. This may be due in part to an incomplete understanding of human immune responses to each disease in its own right [31]. It is appreciated that research of these topics is not a straightforward task; for example, assessing the effect of malaria on MTCT of HIV can involve the use of AIDS patients, asymptomatic patients with normal or lowered CD4+ T cell counts, or areas of stable or unstable malaria transmission, each variable affecting build-up of immunity. There are many factors that can influence outcomes, making it difficult for valid conclusions to be drawn.
Campaigns to improve sexual education in developing countries are starting to show benefits. The US President’s Emergency Plan for AIDS Relief provides funds to lower income nations that have high rates of HIV transmission, coinciding with areas of intense malaria endemicity. It is arguable whether or not the ABC approach (abstinence, being faithful and using condoms) supported by this program has contributed actively to a decline in transmission but, regardless, any form of public health education should be viewed in a positive light [78]. Attendance at antenatal clinics provides an ideal forum at which to educate women about HIV and malaria transmission prevention and prophylaxis, to provide counseling and support, and to offer HIV testing and malaria screening [79]. In isolation, such incremental action on an individual level will not solve the massive challenges of HIV and malaria infections globally, but for an expectant mother it may make a real, potentially life-changing difference, for both her and her child.
Economics plays a key role regarding if, and over how long a time frame, investigations of new therapeutic agents and vaccines can be sustained. Considering financial arguments for and against research development against an ethical perspective, especially where HIV and malaria are concerned, will always spark debate. In a sense, accessibility to money is able to control life and death for the majority of those who suffer from these major human diseases. The contribution of pharmaceutical companies to the overall funding of HIV vaccine development is modest, approximately 10% of total income [80]. This may be because of the poor commercial value of the market, in the sense that those whose need for treatment is greatest cannot afford it. Also, if a prophylactic vaccine for HIV or malaria did become available, the market for drugs in areas where this is administered would decrease significantly.
The existence of poor models for HIV research limits vaccine development as findings from animal studies should be extrapolated only with caution to infection of humans [81]. It would be considered unethical to test a vaccine in a human volunteer before an animal trial, but if results between the two do not correlate the validity of the use of animal models may be questioned. Also, potential use of therapeutic vaccines for both HIV and malaria in pregnancy is difficult to research, again due to lack of reliable animal models to demonstrate safety and immunogenicity [66,82]. A lack of research is apparent concerning drug therapy for both diseases during pregnancy, limiting treatment options for expectant mothers who are in an increased immuno compromised state and who thus have a greater need for medication.
The impact of HIV and malaria infections is a continuing global public health challenge. Co-infection of these major human infectious diseases is thought to affect approximately one million pregnancies per year in subSaharan Africa. Despite this scenario, there is a lack of conclusive evidence regarding the implications of co-infection in a non-pregnant host, never mind a pregnant woman. It has only been over the last decade that interest in co-infection of the pregnant host has attracted significant attention. To date, knowledge of treatment in co-infection is not extensive, limiting current therapeutic options for these patients. Although a vaccine for both HIV and P. falciparum may be considered possible, either is unlikely to be achieved in the near future. A deeper insight into the interactions of antimalarial and anti-retroviral drugs during pregnancy is required so that improved regimens can be used whilst the wait for a vaccine continues.
The authors declare that they have no competing issues of interest.
Download Provisional PDF Here
Article Type: Review Article
Citation: Barker ML, Taylor-Robinson AW (2016) Therapies to Combat Co-Infection of HIV and Malaria during Pregnancy in Sub-Saharan Africa. Int J Vaccine Immunizat 2(2): doi http://dx.doi.org/10.16966/2470- 9948.111
Copyright: © 2016 Barker ML, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Publication history:
All Sci Forschen Journals are Open Access