Table 1: Scheme of BoHV-1 tmv intranasal infection study design in lambs.
Christian T. Lay1 Joan M. Burke2 Daniel B. Paulsen1 Shafiqul I. Chowdhury1*
1Department of Pathobiological Sciences, School of Veterinary Medicine, Louisiana State University, Baton Rouge, LA 70803, USA*Corresponding author: Shafiqul I. Chowdhury, Department of Pathobiological Sciences, School of Veterinary Medicine, Louisiana State University, Baton Rouge, LA 70803, United States, Tel: 225789681; Fax: 225-5789701; E-mail: chowdh@lsu.edu
Bovine herpes virus 1 (BoHV-1) causes respiratory infections and abortions in cattle, and is an important component of Bovine respiratory disease complex (BRDC). BoHV-1 has also been isolated from sheep with respiratory disorder. Experimentally, sheep and goats are infected productively with BoHV-1 after intranasal inoculation and they generate immune response against BoHV-1. We have engineered a triple genemutated bovine herpes virus type 1 (BoHV-1 tmv) vaccine virus which is protective against BoHV-1 in calves. In the present study, we have tested BoHV-1 tmv infectivity and serum neutralizing antibody titers in lambs after intranasal inoculation. The results showed that BoHV-1 tmv replicated well in the nasal mucosa with moderate titers for 7 days and the infected lambs generated serum neutralizing titers.
Bovine herpes virus type 1; Triple gene mutant; Sheep; Intranasal vaccination
Cattle are the natural host of bovine herpes virus type 1 (BoHV-1), however sheep and other small ruminants can be infected with BoHV- 1. In cattle, BoHV-1 causes severe respiratory tract infection known as infectious bovine rhinotracheitis (IBR) and abortion in pregnant cows. In addition BoHV-1 is an important component of the Bovine Respiratory Disease Complex (BRDC) also known as “shipping fever” [1-5]. In sheep, BoHV-1 infection may not cause clinically significant disease; however, it may cause moderate seromucopurulent nasal discharge. Our recently developed, triple-mutant strain of BoHV-1 has substantial promise as a vaccine vector virus. Several viral diseases of sheep that are significant in various parts of the world, such as Rift Valley fever, bluetongue, and border disease, lack effective vaccines. Vector-based vaccines using the triplemutant strain of BoHV-1 may be a viable way to approach these diseases. In cattle, wild type (wt) BoHV-1 evades the cellular immune response and avoids host cytotoxic CD8+ T cell recognition because envelope protein UL49.5 transiently down regulates the cell surface expression of major histocompatibility complex (MHC) class I molecules [6-8]. This allows vigorous virus replication in the nasal and upper respiratory tract epithelial cells and results in pustules and ulcerous lesions [9]. This combined with immunosuppression, facilitates secondary bacterial infection resulting in pneumonia and/or BRDC [4,8,10].
In the absence of secondary bacterial infection, the infected animals usually survive, but become lifelong carriers of BoHV-1. After initial replication in the respiratory mucosa or nasal epithelium, BoHV-1 enters the sensory nerve endings (axon terminals) of the trigeminal nerve in the nasopharynx. Subsequently, the nucleocapsids along with tegument structures are transported retrogradely (up the axon of the trigeminal nerve) to the corresponding neuronal cell body in the trigeminal ganglia (TG), where they establish lifelong latency [3]. Thereafter and during the entire lifespan of the latently infected animal, stress can induce the latent virus to reactivate. This usually leads to its anterograde transport (down the axon, away from the cell body) to the nerve endings in the nasopharynx (a maxillary branch of the trigeminal nerve). This further leads to nasal viral shedding and subsequent viral transmission to susceptible animals, thus enhancing the maintenance and spread of the virus in animal populations [3]. Experimentally, sheep and goats support productive BoHV-1 replication in nasal epithelium. Intranasal infected sheep and goats shed virus in the nasal secretions [11,12]. Several experimental studies have also demonstrated that infected sheep and goats mount an immune response against BoHV-1 following intranasal infection [11,12]. BoHV-1 also establishes latent infection in the trigeminal ganglia of sheep and goats following intranasal infection. Like in cattle, the latent virus reactivates due to stress and results in recurrent virus nasal excretion and seroconversion (rising antibody titers) [11,12].
We have engineered a triple gene-mutated bovine herpes virus type 1 (BoHV-1 tmv) vaccine, which does not down-regulate MHC-I cell surface expression, lacks virulence and is not shed in the nasal excretions following reactivation from latency. This is because in the BoHV-1 tmv, domains within the BoHV-1 UL49.5, that down regulate MHC-I are deleted, the entire cytoplasmic tail sequences of glycoprotein gE important for virulence as well as anterograde
neuronal transport and the entire Us9 coding sequences required for anterograde axonal transport are deleted [13]. Recently, we have determined that following intranasal vaccination/infection of calves, the BoHV-1 tmv is significantly attenuated but replicates efficiently and generates significantly better protective immune responses when compared with a gE-deleted virus that also has anterograde neuronal transport defect and is not shed in the nasal secretions following reactivation from latency [14].
Since wild type (wt) BoHV-1 may infect the sheep intranasally, cause moderate clinical signs and induce serum neutralizing antibody [11], we hypothesized that the BoHV-1 tmv would be attenuated in sheep when administered intransally; However, it would induce a BoHV-1- specific immune response following intranasal infection. If this was true, then BoHV-1 tmv could be used as a viral vector to express protective antigen(s) of other viruses, which infect cattle, sheep and goats, such as Rift Valley fever [15], peste des petits ruminants virus [16], bluetongue [17], and border disease. Here we determined the replication/shedding property of BoHV-1 tmv and serum neutralizing antibody response in sheep following intranasal infection. Our results showed that BoHV-1 tmv infected sheep and induced a BoHV-1-specific serum neutralizing response following intransal infection.
The MDBK cell line used for virus propagation was maintained as described earlier [18]. BoHV-1 tmv virus was constructed earlier [13] using the parental BoHV-1 Cooper strain.
Animal infection, handling, sample collection and euthanasia protocols were previously approved by the LSU Institutional Animal Care and Use Committee. Twelve BoHV-1 and BVDV negative 4-6 months old male lambs (Kathdin breed), weighing approx. 60-80 lbs were selected. Six lambs each were randomly assigned into two rooms at the LSU-AG center large animal isolation facility. Infection of lambs and the sample collection scheme is shown in table 1. The animals in the infected group were inoculated intranasally as described earlier [13] with 1 × 107 plaque forming units (PFU) in 0.5 ml/nostril of BoHV-1 tmv (Total 2 × 107 PFU per sheep). Control uninfected lambs were housed separately, and were inoculated similarly with 0.5 ml /nostril of cell culture media.
For clinical signs, rectal temperatures, nasal discharge and nasal lesions were recorded during each visit and clinical scores were assigned to each parameter. Rectal temperatures were scored 0-4 (<39.0°C, 39.5°C, 40.0°C, 40.5°C, >40.9°C), nasal discharges were scored 0-4 (normal, serous, mild and severe mucopurulent), nasal lesions were scored 0-3 (normal, hyperemia, pustules and ulcers).
For virus titrations MDBK cells were seeded in 24 well plates (Corning) at a concentration of about 3 × 105 cells 500 μl/well a day before. The virus suspension was diluted with DMEM containing 2% FBS (fetal bovine serum) in a series of 10 fold dilutions ranging from 10-1 to 10-7. After decanting the media from the 24 well plates containing cells, 200 μl aliquots from each dilution were added to two corresponding wells and incubated for 2 h in a CO2 incubator at 37°C. Thereafter, 400 μl of of overlay medium, 1.3% carboxy methyl cellulose in DMEM containing 2% FBS (Sigma #C5013), were added to each well and the plates were incubated further for 48 h at 37°C. The cells were fixed with 4% formaldehyde solution for 30 min at room temperature (RT) followed by staining with 0.35% crystal violet solution (15 min).Virus titer was expressed as Plaque forming units (PFUs)/ml by using the following formula.

Table 1: Scheme of BoHV-1 tmv intranasal infection study design in lambs.
=Average no of plaques × Reciprocal of the viral dilution × 5.
For virus–neutralization assays, 24 well plates containing MDBK cells were prepared as above. Serum samples were inactivated for 30 min at 56°C and diluted in DMEM containing 2% medium in a series of twofold dilutions leaving 250 μl of the diluted serum in each tube. Tubes were then incubated for 1 h at 37°C with 250 μl of virus suspension containing 100 PFUs/100 μl. As controls, 5 tubes containing DMEM (250 μl) were incubated as above with 250 μl of virus suspension containing 100 PFUs/100 μl. Thereafter, 200 μl aliquots from each serum/virus dilutions and the controls were added in duplicate to the corresponding wells of the 24 well plates as above and incubated further for 48 h at 37°C. The cells were fixed and stained as above, and the number of plaques in each well was counted. Average number of plaques counted in the control wells were designated as Vo and average number of plaques counted in each serum dilution were designated as V. The percentage of remaining plaques of each serum dilution were calculated by using the following formula in Microsoft Excel =(V*100)/Vo ), which was then plotted to calculate the 50% plaque reduction titer of each serum sample. The titers were expressed as the reciprocal of the highest serum dilution that caused a 50% reduction in the number of plaques relative to the virus control.
Following primary infection/vaccination, clinical scores for the infected lambs were determined. Based on the results (data not shown), the animals had no fever, noticeable nasal lesions nor nasal secretions. Therefore, their clinical scores were not significantly different from shaminfected controls (data not shown). BoHV-1 tmv replicated efficiently in the nasal epithelium and shed virus for up to 7 days post-infection (dpi). The highest yield of the virus in the nasal swabs was obtained at 4 dpi (1-2 × 104 PFUs (Figure 1). Our earlier results of calf vaccination-challenge study also showed that even though BoHV-1 tmv replicated well (2 × 105 – 8 × 105 PFUs) in the nasal epithelium of the infected calves, there were no noticeable clinical signs in the calves (rectal temperature, nasal secretion and nasal lesions [13].
Figure 1: Nasal virus shedding following primary intranasal infection/ immunization of lambs with BoHV-1 tmv. As a control, sham-infected lambs were also tested during primary infection. Nasal swabs were collected at indicated time points in 1 ml of DMEM containing Penicillin/ Streptomycin. Virus titration from the nasal swabs was performed by standard plaque assay. As expected, control sham infected lambs did not shed any detectable amounts of virus.
Earlier, Hage et al. [11] reported that wt BoHV-1, strain lam replicated in the nasal epithelium of sheep and resulted in nasal viral shedding, which lasted up to 15 dpi with a viral yield as high as 107.5 TCID50. In addition, the inoculated sheep had moderate seromucopurulent nasal discharge. In calves, BoHV-1 tmv is attenuated when compared with the parental wild type strain Cooper, both, with respect to duration of nasal viral shedding and clinical signs [13]. In calves, BoHV-1wt and BoHV-1 tmv nasal viral shedding lasted for 12 dpi and 9 dpi, respectively. Notably, BoHV-1 tmv infected calves did not show any clinical signs. In lambs, nasal viral shedding of BoHV-1 tmv lasted for 7 days, which is 9 days shorter than the BoHV-1 wt nasal shedding in sheep reported by Hage et al. [11]. In this study, we did not compare the virus shedding of BoHV-1 wt Cooper strain in the lambs. However, based on our earlier results in calves which used essentially the same methods as this study, we believe that as in calves BoHV-1 tmv showed an attenuated growth phenotype in the lambs [13]. These results are also consistent with the assumption that BoHV-1 tmv showed slightly more attenuated property in the lambs than in the calves.
To evaluate BoHV-1-specific immune response in the lambs vaccinated with BoHV-1 tmv, sera collected on 0, 7, 14 and 21 dpi from the BoHV- 1 tmv-infected and sham-infected sheep (Table 1) were tested for serum virus neutralizing (VN) antibody titers against BoHV-1 wt. As shown in figure 2, lambs infected with BoHV-1 tmv generated VN antibody titers following primary infection and by 21-28 dpi, average serum virus neutralizing titer was 6-8. Earlier, in calves, we obtained a slightly better serum neutralizing titers following intranasal vaccination with BoHV- 1 tmv (SN titers between 15-20). While the serum neutralizing titers obtained against BoHV-1 in the lambs vaccinated with BoHV-1 tmv were lower than in the calves, they may be protective. If not, we believe that by simultaneous intramuscular injection of the vaccine virus one could obtain higher serum neutralizing titers.
In summary, the attenuated BoHV-1 tmv retained its infectivity and immunogenic properties in lamb and thereby has shown its potential for further investigation as a vaccine against BoHV-1 and as a viral vector vaccine. Our currently ongoing research has produced encouraging results suggesting that BoHV-1 tmv can be used as a vector for protective antigens of other bovine respiratory viruses, i.e. bovine viral diarrhea virus (BVDV) envelope protein E2 [18,19], bovine respiratory syncytial virus (BRSV) envelope proteins F and G [20,21]. Furthermore, we believe that it could serve as a vector for delivering protective antigens Gc and Gn of Rift Valley Fever virus (RVFV) in cattle and small ruminants [22,23]. RVFV infection in critical livestock species such as sheep, goats, buffalo and cattle, cause significant economic losses through death, abortion and decreased milk production [24]. In addition, RVFV-infected sheep and cattle are the main source of infection to humans. In 2000, there were 747 human cases in Saudi Arabia, which was likely due to massive movements of animals and people during the Hajj [25]. Currently we are generating a BoHV-1 tmv vectored vaccine against RVFV which, if efficacious, can be used to vaccinate sheep, goats, cattle and perhaps buffalo to prevent future RVFV outbreaks in human.
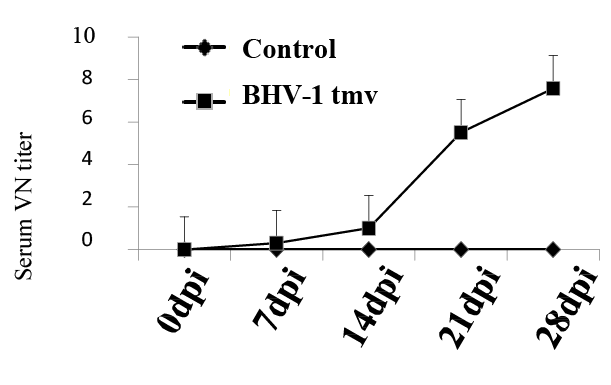
Figure2: Virus neutralizing antibody titers in lambs following primary intranasal infection / immunization with BoHV-1 tmv. Serum samples were collected at indicated time points, inactivated by heating (56°C for 30 minutes) and stored at -20°C. Serum virus neutralization titer was calculated by plaque reduction assay. As expected, control sham-infected lambs were negative for BoHV-1-specific neutralizing antibodies.
This work was supported by USDA grants to S.I. Chowdhury (2009- 35204-05200 and 2007- 35204-17358).
Download Provisional PDF Here
Article Type: Research Article
Citation: Lay CT, Burke JM, Paulsen DB, Chowdhury SI (2016) A Triple Gene Mutant of BoHV-1 Administered Intranasally in Lambs Replicates Efficiently in the Nasal Epithelium and Induces Neutralizing Antibody. Int J Vaccine Immunizat 2(1): doi http://dx.doi.org/10.16966/ 2470-9948.108
Copyright: © 2016 Lay CT, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Publication history:
All Sci Forschen Journals are Open Access