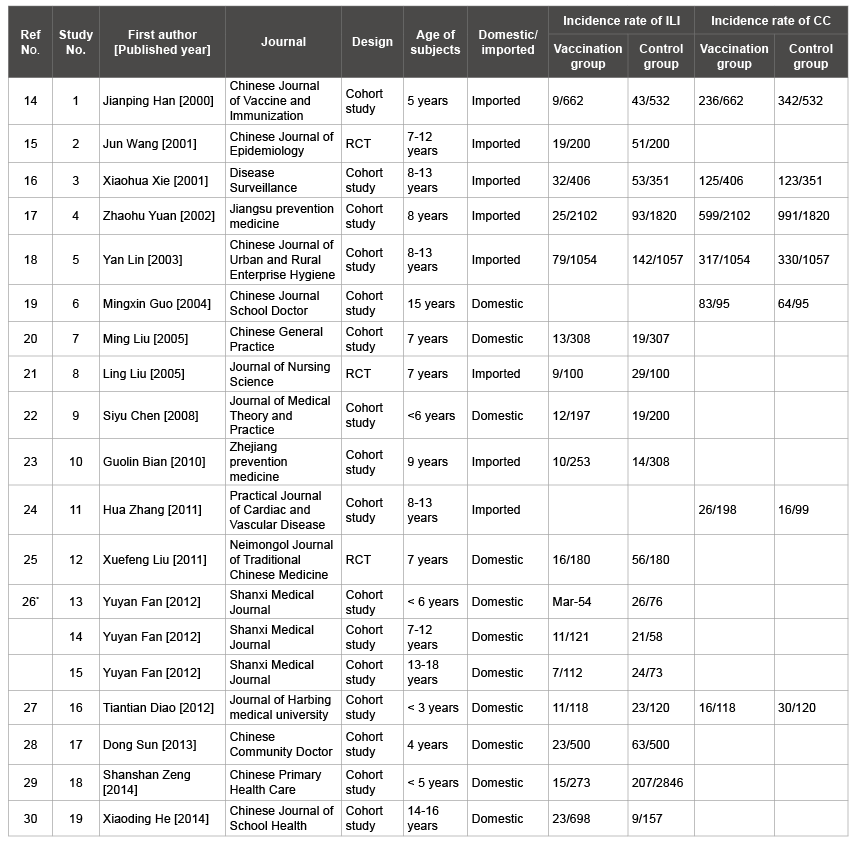
Table 1: Basic information of studies included in this meta-analysis
*
: The article contains more than one study
Qian Li Yu Hu* Bing Zhang Yaping Chen
Institute of Immunization and Prevention, Zhejiang Center for Disease Control and Prevention, No. 3399 Binsheng Road, Binjiang District, Hangzhou, P.R. China*Corresponding author: Yu Hu, Institute of Immunization and Prevention, Zhejiang Center for Disease Control and Prevention. No. 3399 Binsheng Road, Binjiang District, Hangzhou, P.R. China, Tel: +86-571-87115169; E-mail: husix@163.com
Objective: To evaluate vaccine effectiveness (VE) of seasonal inactivated influenza vaccine (SIIV) in Chinese children and adolescent under18 years of age.
Methods: We searched China National Knowledge Internet (CNKI), Wan Fang Database (WF), PubMed, EMBASE and the Cochrane Library for Chinese and English language articles describing VE of SIIV in Chinese children and adolescent under 18 years of age. Meta-analysis, sensitivity analysis, subgroup analysis and publication bias test were performed using Rev Man software, version 5.1 and STATA statistical software, version 11.0.
Results: A total of 19 studies in 17 papers were included. The overall VE of SIIV for influenza like illness (ILI) was 68% (95%CI: 56-76%) in RCTs while that was 59% (95%CI: 46-68%) in cohort studies. The pooled VE of SIIV for ILI varied by age of subjects (VE=61% in subjects aged ≤ 6 years versus 57% in subjects aged 7-18 years) in cohort studies. The pooled VE of SIIV for ILI varied by vaccine types both in RCTs (VE=71% for domestic vaccine versus 49% for imported vaccine) and cohort studies (VE=53% for domestic vaccine versus 60% for imported vaccine).
Conclusions: SIIV provided good protection from ILI in Chinese children and adolescents aged 0-18 years. More studies of VE on SIIV with larger samples are needed for supporting the subgroup analysis in future.
Vaccine efficacy; Seasonal inactivated influenza vaccine; Meta analysis; Children; Adolescents
Influenza A and B viruses can cause seasonal influenza through droplets and aerosols originating from the respiratory secretions of patients or infected people. Although the number of influenza associated deaths declines substantially, it has been estimated that the annual incidence rates are 5%-10% in adults and 20%-30% in children worldwide [1]. Children under 5 years of age, and especially those under 2 years of age, have a higher burden of seasonal influenza than other population. A review estimated that there were 90 million new seasonal influenza cases in 2008 (including 28000-111500 deaths) and majority of deaths from influenza occurred in developing countries [2].
Epidemiologic observation has suggested that children have the highest attack rates of influenza. In two studies from the United States [3,4], a substantial excess of hospital admission rates was observed in healthy children. A substantial burden of morbidity and hospital admission for influenza among children below 15 years has been reported in Honk Hong [5]. In Chinese mainland, there was no accurate data on morbidity and mortality on confirmed seasonal influenza, but the incidence of influenza like illness (ILI) was 13.1%-13.7% from 2001 to 2003 [6].
Seasonal Inactivated Influenza Vaccine (SIIV) consists of three strains representing influenza A (H3N2), A (H1N1), and B viruses and it is generally acknowledged that SIIV is one of the most effective ways to prevent seasonal influenza epidemics [7]. Recommendations on SIIV vary in different countries. SIIV is recommended to the people aged ≥ 50 years, healthy children aged 6-23 months, and other high risk groups in United States, while in most other countries SIIV is generally recommended to the people ≥ 65 years or other high risk groups, but not in children1. In China, SIIV is a Category II (parent-pay) vaccine and is currently recommended to the children aged from 6 months to 5 years, elder population over 60 years of age, persons with specific chronic diseases, health-care workers and pregnant women [8].
There were three published reviews on assessing the vaccine efficacy (VE) in health children [9-11]. These quantitative estimates were relatively similar, but their conclusions were inconsistent. One review expressed a skeptical attitude on universal childhood SIIV vaccination, while the other two reviews considered that SIIV vaccination was a possible option for preventing seasonal influenza among healthy children. However, there were relatively few SIIV VE data from China in the international literature.
To provide a comprehensive overview on this issue, a meta-analysis was conducted to assess the VE of SIIV in Chinese children/adolescents less than 18 years of age, and to provide evidence-based data for improving the SIIV immunization strategy.
In order to include all literatures evaluated the VE of SIIV in Chinese children/adolescents under 18 years of age for this study, we searched China National Knowledge Internet (CNKI) (through January, 2015), Wan Fang Database (WF) (from 1980 through January 2015), PubMed (through January 2015), EMBASE (through January 2015) and the Cochrane Library according to the following strategy: (influenza OR flu) AND vaccine* AND (child* OR adolescent* OR young) as key words in the title/abstract. We reviewed reference lists of each article and implemented manual searches in some relevant journals, such as the Chinese Journal of Vaccines and Immunization [in Chinese], Vaccine and Human Vaccines and Immunotherapeutics. We also contacted the corresponding authors for more details if necessary. Studies published only in English or Chinese were included.
We included published studies on evaluating the VE of SIIV in healthy Chinese children/adolescents under 18 years of age, and we ignored the type of vaccine (domestic/imported), number of doses, sample size. Prospective studies such as randomized controlled trials (RCT), cohort studies, and other observational studies were included. The exclusion criteria of this analysis included studies of methodology, molecular biology, vaccine development, animal studies, popular science lectures, news articles, and reviews. The studies on the live attenuated influenza vaccine were also excluded. When more than one article was based on a same trail data, only the most recent published report was included. Included studies must have a control group that received a placebo or got vaccination other than SIIV, and it should provide sufficient data to calculate the incidence of ILI or common cold (CC), coverage rate, or relative risks/odds ratios (RR/OR) with 95% confidence interval (CI), respectively.
Every included studies was evaluated separately by two researchers who were blind to resources and authors. The information of the included studies towards authors, year of publication, journal, study design, age of subjects, number of vaccine doses, type of vaccine, number of ILI/CC cases in both vaccination and control group were extracted and entered into a prepared table. The Jadad scale [12], which consists 3 items on randomization, masking and withdrawals/dropouts, was adapted to assessing the methodological quality of RCTs. The range of the Jadad scale is from 0 to 5. RCTs with scores of 0-3 and 4-5 were defined as low and high quality in this analysis, respectively. The Newcastle-Ottawa Scale (NOS) [13], which consists 9 items on sample selection, comparability and outcome, was adopted to assessing the methodological quality of non-randomized studies. The NOS assigns a maximum of nine points to each study. Non-randomized studies with scores of 1-3, 4-6, and 7-9 were defined as low, median, and high quality in this analysis, respectively.
The units of this analysis were single comparisons of one treatment versus control group in a influenza season after SIIV vaccination, hence, when more than one treatment was found in a study, every treatment was compared with the relevant control group and included as a separate unit in the meta-analysis. This meta-analysis was also conducted for ILI and CC separately. The χ2 test was used to evaluate heterogeneity among included studies and we considered a P value <0.10 as being significant. We calculated RR for RCTs and cohort studies while OR for case control studies. Pooled estimates of RR/OR with 95% CI were calculated using random effects models if there was significant heterogeneity among included studies. On the contrary, fixed effects models were adopted. VE was defined as (1-RR) ×100%, and similarly for the OR. Since studies with different design had different strength of evidence, pooled estimates were presented separately. Studies with lower quality were included in the sensitivity analysis. We implemented subgroup analysis using the random effect model to explore reasons of heterogeneity according to the following two factors:
(1) Age of subjects- Persons aged 0-6 years were categorized as group 1 while persons aged 7-18 was categorized as group 2.
(2) Type of vaccine (domestic/imported)- Begg’s test and Egger’s test was used to evaluate the publication bias.
Meta-analysis, sensitivity analysis, and subgroups analysis were performed by RevMan software, version 5.1. Publication bias evaluations were performed by STATA statistical software, version 11.0.
A total of 3409 articles were identified from the literature search process. After screening the titles and abstracts, we excluded 3357 articles as they were duplicate or irrelevant. 35 articles were also excluded as they did not provide insufficient data. Finally, 17 articles [14-30], involving 19 studies, were included in this meta-analysis (one article contained three different studies). All the included studies were published in Chinese. All the 19 studies referred to one dose. Of the 19 studies, 3 were RCTs and 16 were cohort studies. Two of the three RCTs included children aged 7 years and the other included adolescents aged 7-12 years. One RCT used domestic SIIV and the others used imported SIIV. Six cohort studies included children aged 0-6 years and the others included adolescents aged 7-18 years. Ten cohort studies used domestic SIIV and the others used imported SIIV (Table 1). All the included RCTs articles were of high quality. Among articles of cohort studies, seven were high quality, six studies were median quality and one study was low quality (Table 2). All studies used only one vaccine type and the following up period lasted for at least one year.

Table 1: Basic information of studies included in this meta-analysis
*
: The article contains more than one study
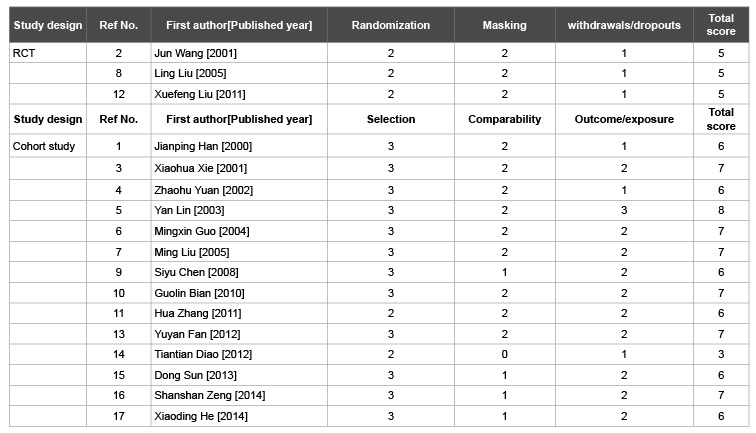
Table 2: Results of the quality assessment using the Newcastle-Ottawa Scale (NOS)
Notes: The quality of RCTs was assessed by Jadad scale (the maximum points forrandomization is 2, for masking is 2, for withdrawals/dropouts is 1 and
for total score is 9); the quality of cohort studies was assessed by NOS scale (the maximum points for selection is 4, for comparability is 2, for outcome is
3 and for total score is 9).
Among RCTs for ILI, pooled estimates of RR was 0.32(95%CI: 0.24-0.44) and the pooled estimates of VE was 68% (95%CI: 56-76%) (Figure 1). Among cohort studies for ILI, pooled estimates of RR was 0.41 (95%CI: 0.32-0.54) and the pooled estimates of VE was 59% (95%CI: 46-68%) (Figure 2). Among cohort studies for CC, pooled estimates of RR was 0.76 (95%CI: 0.56-1.03) (Figure 3).
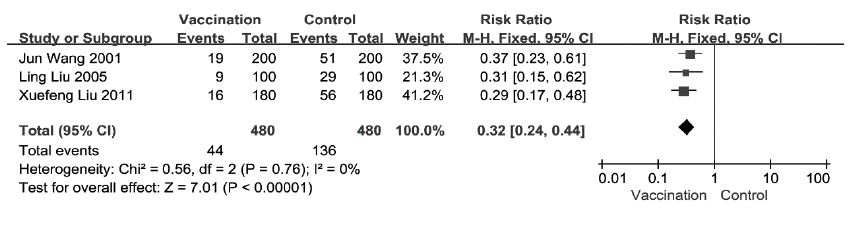
Figure 1: Comparison of VE of SIIV for RCT studies (Outcome: ILI)
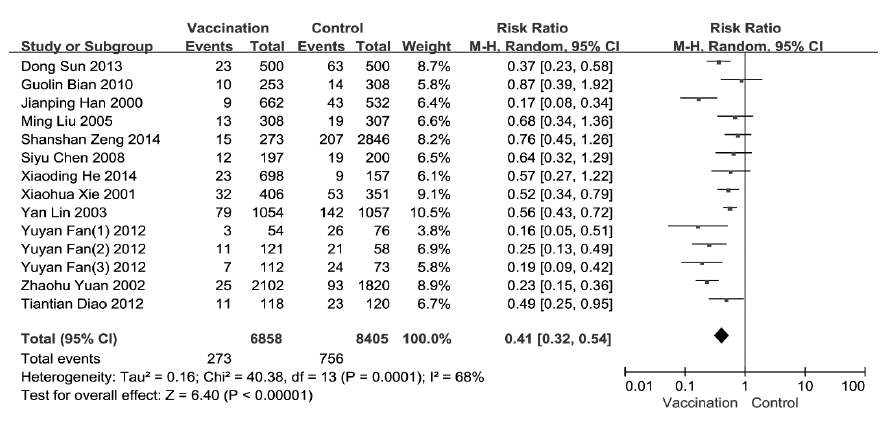
Figure 2: Comparison of VE of SIIV in cohort studies (Outcome: ILI)

Figure 3: Comparison of VE of SIIVin cohort studies (Outcome: CC)
Sensitivity analysis was conducted according to the total NOS score. Through deleted the study with total score of 3, the pooled RR for ILI among the remaining thirteen cohort studies was 0.4 (95% CI: 0.31-0.55) and Z statistic was 6.09 (P<0.00001). The pooled RR for CC among the remaining six cohort studies was 0.79 (95% CI: 0.57-1.10) and Z statistic was 1.38 (P=0.17). The relevant pooled estimates were not substantially different.
In cohort studies, the pooled estimate of RR for ILI was 0.39(95% CI: 0.24-0.64) among persons aged ≤ 6 years and 0.43(95% CI: 0.30-0.61) among persons aged 7-18 years. The pooled estimate of RR for CC was 0.55(95% CI: 0.49-0.62) among persons aged ≤ 6 years and 0.86(95% CI: 0.57-1.29, P=0.47) among persons aged 7-18 years. We did not conduct the subgroup analysis in RCTs as all these studies were implemented among persons aged >6years (Table 3).
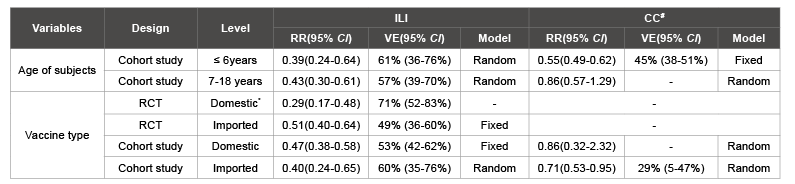
Table 3: Results of subgroup analysis on VE of SIIV
#: There was no included RCT study using CC as the outcome; *: Only one study included.
In RCTs, the pooled estimate of RR for ILI was 0.29(95% CI: 0.17-0.48) for domestic vaccine while 0.51 (95% CI: 0.40-0.64) for imported vaccine. In the cohort studies, the pooled estimate of RR for ILI was 0.47 (95% CI: 0.38-0.58) for domestic vaccine while 0.40 (95% CI: 0.24-0.65) for imported vaccine; the pooled estimate of RR for CC was 0.86 (95% CI: 0.32-2.32, P=0.77) for domestic vaccine while 0.71 (95% CI: 0.53-0.95) for imported vaccine.
Potential publication bias for both two outcomes (ILI and CC) was evaluated by Begg’s funnel plot. The Begg’ s test results showed no significant publication bias as the P values were 0.174 for ILI and 0.368 for CC, respectively. Similarly, Egger’s test results showed no evidence for significant publication bias as the P value was 0.105 for ILI and 0.294 for CC, respectively (Figure 4).
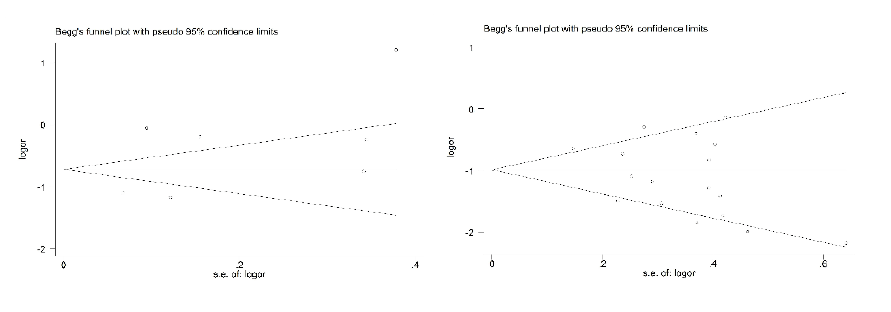
Figure 4: Begg’s Funnel plot of included studies of SIIV (outcome: ILI for left figure and CC for right figure)
The VE is one of most necessary and valuable evidence for formulating the vaccination strategies. As the VE of SIIV varied across different population and areas, implementation of systematic, standard meta-analysis can provide scientific evidence for developing immunization strategy. This meat-analysis involving over 16000 participants aged 0-18 years provided definitive quantitative evidence of SIIV in children and adolescents in China.
The VE of SIIV among healthy children had been evaluated by three meta-analysis and the estimates of VE against ILI ranged from 28%-45% [9-11]. Although the estimates of VE of SIIV for ILI were substantially concordant, the authors gave contrasting interpretations due to the relevant methodological issues (outcome definition, criteria of study inclusion or exclusion). The overall estimates of the VE of SIIV in this study were 59% for ILI among RCTs and 68% for ILI among cohort study. The possible reason for difference of VE compared with previous reports was that the pooled estimates were from more trails and subjects than previous reviews. Compared with high VE of other vaccines such as varicella vaccine or measles containing vaccine, the lower VE of SIIV for ILI may be attributed to the fact that many pathogens other than influenza virus may confuse the clinical diagnosis and some of the clinically confirmed cases could not be prevented even by a SIIV even with totally efficacious [31]. Unfortunately, we could not estimate the VE of SIIV for laboratory confirmed cases because these data were not available yet.
Given the wide disparities caused by potential impact factors like study design, settings, age of subjects and other potential impact factors not included in this analysis such as disease causing viral strains or matching of the vaccine to the circulating strains, it would be reasonable that there was a significant heterogeneity among included studies. As the heterogeneity indicated, we carried out the subgroup analysis to explore sources of variation. For example, differences were seen by age of subjects and imported/domestic vaccine. The estimate of pooled VE for persons aged ≤ 6 years was relatively higher than that for persons aged 7-18 years while it had been indicated that the VE was greater in elders than in younger ones [32]. The main reasons for our opposite results may include the waning of immunity, the matching of the vaccine to the circulating strains, and the elder ones vaccinated ≤ 6 years of age without any boost in recent years.
Besides the potential source of heterogeneity like age of population, we conducted additional stratified analysis to assess the type of vaccine on VE estimates of SIIV. The estimate of VE for imported SIIV was lower than that for domestic SIIV in RCTs while the estimate of VE for imported SIIV was higher than that for domestic SIIV in cohort studies. These differences might be due to the differences in study design, production of the vaccines. The results from RCTs would be more stable and reliable that cohort studies in general, but there was only three RCTs included in this analysis, especially only one RCT for domestic vaccine and it was too scanty to allow a meaningful analysis. These findings must be interpreted cautious lyas the included articles in this subgroup was limited. Additional VE study on SIIV using RCT design is needed to make that determination. The above impact factors in our subgroup analysis would have valuable implications in practice and the field work as they were key elements in developing or improving the SIIV vaccination strategy.
There were some limitations in this study. First, the number of articles that fit for the inclusion criteria was limited. Secondly, important information such as laboratory confirmed cases of influenza, the level of matching between the vaccine used and circulating strains could not obtain from the original articles, which would potentially impact the pooled estimates. Finally, most of the included studies in this analysis were cohort studies. Since the evidence level from RCT was stronger than that from cohort study, there was some potential bias as the majority of the included articles were cohort design.
In conclusion, this study indicated that SIIV provided good protection from ILI among Chinese children and adolescents aged less than 18 years. More researches of VE on SIIV with larger samples are needed for supporting the subgroup analysis in future.
The authors declare that they have no competing interests.
Conceived and designed the study: QL, YH. Literature search: YH, BZ. Data extraction and quality assessment: BZ, YC. Meta-analysis: YH, QL. Wrote the manuscript: QL, YH.
Download Provisional PDF Here
Artiicle Type: Research Article
Citation: Li Q, Hu Y, Zhang B, Chen Y (2016) Vaccine Efficacy of Seasonal Inactivated Influenza Vaccine Using in Chinese Children and Adolescent Under 18 Years of Age: A Meta-Analysis. Int J Vaccine Immunizat 2(1): doi http://dx.doi.org/10.16966/2470-9948.107
Copyright: © 2016 Li Q, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Publication history:
All Sci Forschen Journals are Open Access