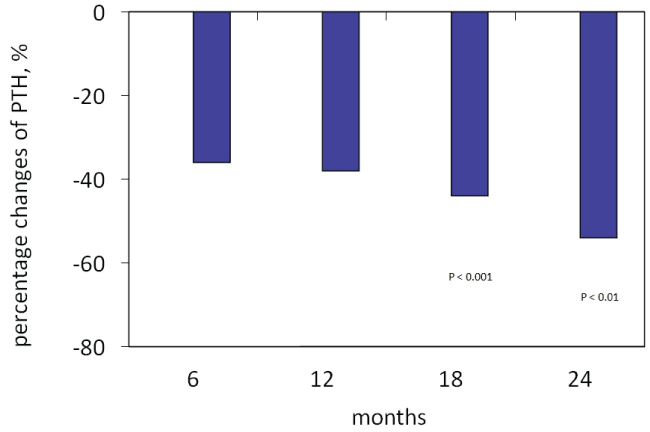
Figure 1: Percentage changes in PTH levels during paricalcitol treatment

Carlo Massimetti* Gea Imperato Sandro Feriozzi
Centro di Riferimento di Nefrologia e Dialisi, Ospedale Belcolle, Viterbo, Italy*Corresponding author: Carlo Massimetti, Centro di Riferimento di Nefrologia e Dialisi, Ospedale Belcolle, Viterbo, Italy, Tel: +3934478787594; E-mail: cmassimetti@libero.it
Normocalcemic secondary hyperparathyroidism may persist in 30–50% of renal transplant recipients (RTRs) at 1 year from kidney transplantation. This can be responsible for: greater loss of bone mass; an increased fracture risk; hypercalcemia, with the risk of nephrocalcinosis and a possible loss of renal function, and vascular calcification; resorting to parathyroidectomy. The aim of the study was to evaluate the effectiveness and safety of paricalcitol on long-term treatment of normocalcemic persistent secondary hyperparathyroidism in RTRs.
The primary outcome of the study was to reduce serum parathyroid hormone (PTH) levels ≥ 50% from baseline. The secondary outcome was to evaluate changes in renal function.
With this purpose, we prospectively selected 24 RTRs with high PTH levels. The main selection of enrollment criteria for this study was: renal transplant vintage ≥ 12 months; increased PTH levels for at least 6 months; serum calcium ≤ 10.2 mg/dl; serum phosphate<4.5 mg/dl; renal function, assessed by eGFR, steadily >15 ml/min/1.73 m2 . Treatment with paricalcitol was started at a mean dose of 1 µg/day, and reduced to 1 µg every other day when sCa levels were>10.2 mg/dl and/or sPO4>4.5 mg/dl. Paricalcitol administration was temporarily stopped when sCa levels were >11 mg/dl.
Every 6 weeks were evaluated: sCr, eGFR, sCa, sPO4, total alkaline phosphatase, FECa, FEPO4, proteinuria, cyclosporine and tacrolimus serum levels. Serum parathyroid hormone levels were assessed every three months. After 24 months of paricalcitol treatment, there was a significant reduction of serum parathyroid hormone levels from 229 ± 164 pg/ml to 88 ± 36 pg/ml (P<0.001), and of alkaline phosphatase from 99 ± 36 mU/ml to 80 ± 22 mU/ml (P<0.05). Serum calcium levels showed a modest trend to increase with just only two episodes of hypercalcemia that required a temporary interruption of paricalcitol treatment and two episodes of isolated hypercalciuria that required reduction of paricalcitol dose to 1 µg every other day. Throughout the study renal function was stable in all patients, including those treated with calcineurin inhibitors (CNIs), and their serum levels remained stable without any need to change their dosages. Blood pressure was meanly well controlled and there were no changes in antihypertensive therapy. Proteinuria showed a modest and not significant reduction. In conclusion, our results suggest that long-term paricalcitol therapy is effective and safe in the control of secondary hyperparathyroidism in RTRs with minimal effects on calcium and phosphorus metabolism, without affecting renal function.
Secondary hyperparathyroidism (SHPT) is a very frequent complication in renal transplant recipients (RTRs). In fact, it is estimated that in about 30-50% of RTRs at one year after renal transplantation (RTx) serum parathyroid hormone (PTH) levels remain high [1]. Post-transplant persistent SHPT has been associated with a high bone turnover, responsible for bone mass loss and a higher risk of fracture [2,3], and progression of vascular calcification [4]. Furthermore, persistent SHPT in 10-40% of RTRs is associated with serum calcium levels at the upper normal limits or hypercalcemia, in particular during the first year of post-RTx [1,5]. High serum calcium levels can contribute to a greater risk of calcification of the graft and to a reduced renal function [6], as well as to reduced patient and graft survival [7]. The important consequences of persistent SHPT have persuaded many authors to take into account an earlier treatment of it, which is not done regularly yet [6,7].
Progressive increase of PTH levels above the normal range could be an indication to start medical therapy, which currently consists of the use of calcitriol, vitamin D analogs, and in selected cases calcimimetics [8].
In severe SHPT patients have to be submitted to parathyroidectomy, which currently is carried out in a 2-5% of RTRs [9]. Nevertheless, some of this treatment are burdened by the risk of side effects. In particular, the use of calcitriol in patients with chronic kidney disease stage 3-5 was associated with an increased risk of hypercalcemia and hyperphosphatemia [10,11], which may be responsible for an increased risk of vascular calcification and cardiovascular mortality [12]. Moreover, calcitriol treatment in RTRs was associated with a significant increase in urinary calcium, that if extended over time can be responsible for nephrocalcinosis [13]. The use of cinacalcet has been reported to be associated with a significant reduction of parathyroidectomy, unfortunately, in our country, the use of cinacalcet in RTRs is reserved for cases of hypercalcemia SHPT (sCa>10.5 mg/dl). Moreover, the use of cinacalcet may increase urinary calcium excretion which may cause nephrocalcinosis in the long term period followed by a worsening of renal function. [14]. Cinacalcet may potentially interfere with calcineurin inhibitors metabolism, which are the immunosuppressive drugs most frequently used in RTRs, this interaction appears to have minor clinical relevance, however, it is advisable to monitor renal function due to the observed decrease in renal function [15]. Surgical parathyroidectomy should be indicated to selected patients and after a period of observation: at least 12 months after RTx [8]. In the early months, post-RTx medical therapy might be sufficient to control serum PTH levels because SHPT may resolve spontaneously, even though at unpredictable times [16]. Furthermore, surgical parathyroidectomy may be associated with worsening of renal function, and SHPT may recur from remaining gland tissue, even after so–called total parathyroidectomy [16,17].
Paricalcitol is a selective activator of vitamin D receptor that has demonstrated a significant improvement of SHPT in patients with chronic kidney disease while inducing less hypercalcemia and hyperphosphatemia than calcitriol, probably due to a reduced effect on intestinal absorption of calcium and phosphorus, this has made its use more manageable than that of calcitriol [18]. Paricalcitol was effective in the control of SHPT, both in stages 3-4 chronic kidney disease [19], as in dialysis [20]. Paricalcitol has also proven effective in cases of SHPT resistant to treatment with calcitriol [21], allowing a more rapid reduction in PTH levels with fewer hypercalcemic episodes [20]. The use of paricalcitol has also been associated with improved survival in dialysis patients [22]. To date, studies on paricalcitol in the treatment of secondary hyperparathyroidism in the RTx are quite different from each other, with regard to the inclusion criteria, PTH levels, paricalcitol doses and duration of the therapy [23- 26]. Therefore we wanted to evaluate the efficacy and safety of relatively low dose of paricalcitol in the long term treatment of SHPT in a group of RTRs.
In the period between January 2011 and December 2014, we enrolled consecutively 24 RTRs with SHPT as defined by K/DOQI guidelines [27]. The study lasted 24 months. Inclusion criteria for paricalcitol treatment were: SHPT diagnosed at least six months prior to study enter; adults (>18-years old); RTx age ≥ 12 months; absence of acute rejection episodes in the last 6 months; serum calcium<10.2 mg/dl; serum phosphorus<4.5 mg/dl; estimated glomerular filtration rate (eGFR), calculated with the formula CKD-EPI [28], steadily>15 ml/min/1.73m2 ; no concomitant therapy with bisphosphonates and/or vitamin D.
The primary study endpoint was the reduction of serum iPTH ≥ 50% from baseline. The secondary study endpoint was to maintain stable the eGFR during the study.
The initial dose of paricalcitol was determined based on the levels of serum calcium and PTH, the maximum dose was established in 1 µg day. When the serum calcium increased over the 10.5 mg/dl and the phosphorus over 4.5 mg/dl paricalcitol dosage was halved, if the serum calcium concentration exceeded 11.0 mg/dl the administration of paricalcitol was temporarily stopped. The dosage of paricalcitol was also revised if there was significant and sustained increased in fractional excretion of calcium (FECa>0.020%) [29], even though serum calcium levels were normal.
The routine laboratory examinations included determination of eGFR, serum calcium and serum phosphorus every 6 weeks and that of the intact PTH (n.v. 9-63 pg/ml) and total alkaline phosphatase approximately every 3 months. Every 6 weeks in the 24-hour urine were quantified creatinine, calcium, phosphorus, and proteinuria. Urinary calcium was expressed as 24-hour calciuria and FECa, calculated by the formula (24- hour calciuria/serum calcium)/(24-hour creatinuria/serum creatinine) × 100. Urinary phosphate was expressed as 24-hour phosphaturia and fractional excretion of phosphate (FEPO4 %), calculated by the formula (24-hour phosphaturia × serum creatinine)/(serum phosphorus × 24- hour creatinine) × 100. Proteinuria was expressed in g/24-hour. They were also regularly monitored for serum levels of cyclosporine and tacrolimus. Data are expressed as the mean ± standard deviation. The t-test for paired data was performed when appropriate. The correlation coefficients were calculated using the Pearson test. For all tests, P<0.05 were considered to be statistically significant. The statistical analysis was performed with SPSS (Statistical Package for Social Science, 11.0, 2003; SPSS Inc., Chicago, IL, USA).
Table 1 shows the main baseline clinical features of the RTRs group. Baseline PTH levels were above two times the upper normal limits in 22/24 patients and between 80 and 120 pg/ml in the remaining 2 patients. Both FECa and FEPO4 were on average high. According to guidelines KDOQI [27], vitamin D levels were on average at the limits between insufficiency and deficiency. Proteinuria was on average less than 0.50 g/24-hour in most patients, resulting higher than 0.50 g/24-hour in just 6/24 of patients treated. The blood pressure was well controlled. Parathormone levels showed a rapid and significant reduction already during the first 6 months of therapy (Table 2). The reduction trend was maintained throughout the observation period, becoming highly significant at 24 months, with a percentage reduction at 24 months baseline of 54% (Table 2 and Figure 1). The percentage reduction of PTH levels at follow-up was more than 50% in 18 patients out of 24. Patients in which the reduction of PTH levels at follow-up was less than 50%, when compared with patients who showed a reduction percentage of PTH levels>50%, were characterized before RTx by higher PTH levels (626 ± 719 pg/ml vs. 341 ± 145 pg/ml; P <0.01); higher levels of phosphorus (6.6 ± 0.6 mg/dl vs 4.9 ± 1.3 mg/ dl; P=NS); a greater use of cinacalcet and vitamin D receptor activators (43% vs 25%, and 71% vs 25%, respectively). Serum total alkaline phosphatase levels decreased significantly (Table 2), in 5 patients with baseline alkaline phosphatase values above the normal ranges normalized. In the first 6 months of therapy, calcium levels showed a progressive increase without ever reaching statistical significance, after that the values stabilized (Table 2). Episodes of hypercalcemia, values>10.5 mg/ dl, which led to the reduction of the dose paricalcitol were a total of five, while those with serum calcium>11.0 mg/dl which required temporary stopped of paricalcitol administration were two. There was no drop-out during the follow-up. In the first three months of paricalcitol treatment, FECa showed an increase that did not reach statistical significance, not shown in the table, after that values decreased back to baseline values (Table 2). In eighteen laboratory measurements, the FECa showed such an increase that required an adjustment of the paricalcitol dosage. In some cases, the increase of the FECa preceded that of the serum calcium. The reduction of FEPO, although not statistically significant, recorded for almost the entire duration of the treatment with paricalcitol did not associate a significant increase in phosphoramide (Table 2). The renal function remained unchanged throughout all the study period (Table 2). Proteinuria showed a slight but no significant decrease (Table 2). However, the reduction of proteinuria was more evident in the group of patients not treated with CNIs (n=5), from 0.54 ± 0.62 to 0.26 ± 0.07 g/24 h (P=NS), than in the group treated with CNIs (n=19), from 0.59 ± 0.62 to 0.40 ± 0.65 g/24 h (P=NS). This differential reduction of proteinuria was independent of the use of drugs that inhibit the renin-angiotensinaldosterone system (RAAS). During the study, there was no significant change of cyclosporine and tacrolimus serum levels that required the need to modify their dosages (Table 2). No clinical side effects attributable to paricalcitol were observed, a temporary interruption occurred only in the presence of severe hypercalcemia, proving overall well tolerated. Also during the study period, there were no recorded episodes of acute rejection or deterioration of renal function.

Figure 1: Percentage changes in PTH levels during paricalcitol treatment
| Age, y/o | 57 ± 12 | FECa, % | 0.018 ± 0.011 |
| Gender (M/F) | 18/6 | Urinary phosphorous, mg/24 h | 771 ± 352 |
| Dialysis vintage, months | 25 ± 24 | FEPO4 , % | 41.5 ± 21.8 |
| Graft vintage, months | 91 ± 86 | Proteinuria, g/24 h | 0.58 ± 0.61 |
| Serum creatinine, mg/dl | 1.8 ± 0.7 | Systolic Blood Pressure, mmHg | 133 ± 13 |
| eGFR, mL/min/1.73 m2 | 46 ± 26 | Diastolic Blood Pressure, mmHg | 77 ± 7 |
| Serum calcium, mg/dl | 9.4 ± 0.5 | Mean Blood Pressure, mmHg | 96 ± 7 |
| Serum phosphorus, mg/dl | 2.9 ± 0.5 | Steroids, mg/d (n° pts) | 5.1 ± 2.2 (18) |
| PTH, pg/ml | 226 ± 167 | Cyclosporin, n° pts | 10 |
| total ALP, mU/ml | 98 ± 36 | Tacrolimus, n° pts | 10 |
| 25OHD3 , ng/ml | 16.4 ± 7.1 | RAAS blocking agents, n° pts | 8 |
| Albumin, g/dl | 3.8 ± 0.3 | CCBs, n° pts | 9 |
| Urinary calcium, mg/24 h | 116 ± 63 | Diuretics, n° pts | 8 |
Table 1: Main baseline clinical characteristics
| Months | -6 | 0 | 6 | 12 | 18 | 24 |
| sCr, mg/dl | 1.8 ± 0.7 | 1.8 ± 0.7 | 1.8 ± 0.8 | 1.8 ± 0.9 | 1.9 ± 1.0 | 1.8 ± 1.0 |
| eGFR, ml/m’/1.73 m2 | 45 ± 22 | 46 ± 26 | 45 ± 21 | 46 ± 25 | 44 ± 21 | 46 ± 23 |
| sCa, mg/dl | 9.4 ± 0.4 | 9.4 ± 0.5 | 9.7 ± 0.5* | 9.7 ± 0.5* | 9.7 ± 0.5* | 9.6 ± 0.4 |
| sPO4, mg/dl | 2.8 ± 0.5 | 2.9 ± 0.5 | 3.2 ± 0.8 | 3.0 ± 0.7 | 3.0 ± 0.6 | 2.9 ± 0.6 |
| Ca x PO4, mg2/dl2 | 26 ± 5 | 28 ± 5 | 30 ± 7 | 29 ± 7 | 29 ± 6 | 28 ± 6 |
| PTH, pg/ml | 198 ± 146 | 229 ± 164 | 139 ± 83* | 140 ± 103* | 124 ± 87^ | 88 ± 36° |
| PTH reduction, % | 91 ± 36 | 99 ± 36 | -36 | -38 | -44 | -54 |
| ALP, mU/ml | 13.0 ± 1.7 | 13.3 ± 1.4 | 84 ± 28 | 82 ± 23 | 76 ± 23^ | 80 ± 22* |
| Hb, g/dl | 118 ± 90 | 117 ± 62 | 13.3 ± 1.6 | 13.2 ± 1.7 | 13.3 ± 1.4 | 13.2 ± 1.4 |
| Urinary Ca, mg/24 h | 0.016 ± 0.012 | 0.018 ± 0.011 | 138 ± 82* | 135 ± 84 | 136 ± 103* | 129 ± 78 |
| FECa, % | 688 ± 284 | 761 ± 349 | 0.21 ± 0.14 | 0.020 ± 0.019 | 0.020 ± 0.020 | 0.021 ± 0.014 |
| Urinary PO4, mg/24 h | 34 ± 17 | 41 ± 22 | 689 ± 259 | 699 ± 358 | 706 ± 305 | 685 ± 295 |
| FEPO4, % | 0.49 ± 0.56 | 0.58 ± 0.61 | 32 ± 12 | 32 ± 12 | 30 ± 14 | 37 ± 14 |
| Proteinuria, g/24 h | 133 ± 13 | 132 ± 13 | 0.51 ± 0.60 | 0.45 ± 0.47 | 0.28 ± 0.10 | 0.33 ± 0.11 |
| Systolic BP, mmHg | 79 ± 6 | 77 ± 7 | 129±13 | 130 ± 14 | 132 ± 13 | 130 ± 15 |
| Diastolic BP, mmHg | 97 ± 6 | 96 ± 7 | 75 ± 7 | 74 ± 5 | 75 ± 7 | 76 ± 8 |
| Mean BP, mmHg | 8 | 8 | 93 ± 8 | 93 ± 7 | 94 ± 8 | 94 ± 9 |
| ACEi/AT1-b | 93 ± 46 (10) | 95 ± 49 (10) | 8 | 8 | 9 | 9 |
| CsA, ng/ml (n° pts) | 6.2 ± 2.7 (10) | 6.6 ± 2.2 (10) | 118 ± 76 (10) | 83 ± 37 (10) | 85 ± 45 (10) | 113 ± 60 (10) |
| Tac, ng/ml (n° pts) | 5.9 ± 1.7 (10) | 5.9 ± 1.9 (10) | 4.6 ± 1.1 (10) | 6.2 ± 2.59 (10) | ||
| Paricalcitol, µg/d | 1.0 ± 0.0 | 0.9 ± 0.2 | 0.7 ± 0.2 | 0.6 ± 0.2 |
Table 2: Main clinical changes during paricalcitol treatment
vs baseline: *P<0.05; ^P<0.01; ᴼ P<0.001
Discussion
In this prospective study, we have observed, over a sufficiently long period of 24 months, the effectiveness of paricalcitol in reducing PTH levels in a group of RTRs with stable renal function and with high levels of PTH during 6 months prior to enrollment in the study. In 76% of patients already 6 months after the beginning of paricalcitol therapy, PTH levels decreased>50% of baseline values. In 91% of the patients, 22/24, PTH levels were reduced to less than 120 pg/ml, representing twice of our high-normal range. These results occurred in the presence of modest changes in serum calcium and phosphorus, and urinary calcium. Significant reduction in PTH levels was achieved by using relatively low dosages of paricalcitol (1 µg/day or every other day). We can not rule out, though unlikely, that the reduction in PTH levels may be partly due to a spontaneous reduction in parathyroid activity that normally occurs within the first 12 or more months from RTx. In fact, all of our patients had a RTx age of ≥ 12 months. Tolerability and safety of the paricalcitol were good since cases of dose reduction or temporary interruption of its administration were always due to the finding of modest hypercalcemia or hypercalciuria. The secondary study endpoint was to evaluate the impact of paricalcitol treatment on renal function. The renal function remained stable during the whole duration of the study. Throughout the study, CNIs serum levels did not change significantly, and these did not require the need to adjust their dosage.
All studies, albeit of limited numbers, that evaluated the efficacy and safety of paricalcitol in the treatment of SHPT proved to be positive [23- 26]. However, their results are not easy to assess because the inclusion criteria were quite different. Particularly, in some of them, the treatment was started immediately after transplantation with PTH values slightly higher. Significant differences were seen with regard to paricalcitol doses used and to the duration of follow-up (in some cases only 6 months).
This has probably contributed to condition the effects of paricalcitol on PTH levels, and especially to determine the differences in the percentage of hypercalcemia and hypercalciuria episodes recorded. Especially hypercalcemia and hypercalciuria when protracted may be associated with adverse clinical consequences both for the transplanted kidney that for some outcomes of the patient [6,7,30]. In the study of Amer et al. [23], for example, they recorded a significant increase in serum calcium and urinary calcium with a fair number of hypercalcemia and hypercalciuria episodes. In fact in this study, unlike what happened in the few other studies [24-26], paricalcitol treatment was started at a fixed dose of 2 µg daily, just only three days after RTx and for PTH values even slightly above the upper normal limits. In this study, PTH levels in the treated group were reduced significantly, but a similar reduction, although less significant, was also recorded in the untreated control group. During the first year of post-RTx PTH levels can be significantly reduced even in the absence of therapies [1], and it also has a significant reduction of bone turnover which only in part is attributable to the reduction in PTH levels [31]. Therefore, especially in the early stages of post RTx, it would seem appropriate to use the paricalcitol not at high doses, and particularly not in patients with PTH values that are slightly above the high-normal range, as it was done in the majority of studies that have evaluated the impact of paricalcitol treatment in the SHPT of post RTx [24-26]. The use of high doses of paricalcitol exposes not only to the risk of hypercalcemia [23,26] but also to the development of adynamic bone disease [32]. The dosage employed is not of secondary importance, in fact 40% of patients with RTx treated with calcitriol at a dose 0.25 µg day, the equivalent of 1 µg of paricalcitol day [33], developed a bone histomorphometric pattern a dynamic like [32]. Nowadays there are no studies in RTRs that have assessed bone turnover during therapy with paricalcitol. However, in the study of Trillini et al. [26] the control of SHPT was associated with a decrease in serum levels of some biomarkers of bone formation and resorption, deemed useful in monitoring bone turnover. This picture may suggest a reduction of elevated bone turnover that characterizes the SHPT in the 25%-50% of RTRs [34]. In our patients, we observed a significant reduction of total alkaline phosphatase serum levels, and it is known that its increase, when combined with that of the PTH levels is almost always associated with the presence of a high turnover bone disease [35]. From the above, it can be deduced that the low number of hypercalcemic episodes reported in our study was due to several reasons. First of all, we used low doses of paricalcitol, monitoring its dosage by measuring not only serum calcium but also urinary calcium. In fact, the increase of calciuria often precedes that of the serum calcium [36]. The second reason is that we treated only RTRs with high PTH levels associated with alkaline phosphatase values high or in the high normal range, this combination very often exclude an underlying bone disorder like adynamic bone disease [35], which is one of the most frequent causes of hypercalcemia especially in the presence of vitamin D receptor activators [37]. The use of paricalcitol in patients with stage 3-4 CKD was associated with a reduction of the renal function evaluated by eGFR [38]. However, these results have not been confirmed when glomerular filtration rate was measured directly using techniques such as the clearance of inulin or iothalamate [23]. In our study renal function was assessed by determination of serum creatinine levels and of the eGFR values, calculated by the CKD-EPI formula [28], and it did not change throughout the entire duration of the study. Although measurement of renal function by eGFR formula is not an accurate method than the one measured by radioisotopic filtration markers, it has been demonstrated that eGFR estimated by CKD-EPI formula is quite close to the glomerular filtration rate measured with 125I-iothalamate [39].
The usefulness of CNIs in preventing rejection of transplanted organ is widely confirmed. However, their use is limited by their potential nephrotoxicity. The cyclosporine kidney injury is mainly characterized by arteriolar hyalinosis, tubular atrophy, interstitial fibrosis and glomerulosclerosis [40]. The rate of CNIs nephrotoxicity is quite variable in literature, but it is very frequent. In a study of 120 kidneypancreas transplant recipients, after 10 years of calcineurin inhibition nephrotoxicity was almost universal, even in grafts with excellent early histologic findings [41]. Tubulointerstitial and glomerular damage, once established, was irreversible, resulting in declining renal function and graft failure. Experimental works have shown that the use of paricalcitol is able to reduce the inflammatory and profibrotic effects induced by the use of cyclosporine [42]. There are experimental, but non-clinical studies, demonstrating the efficacy of paricalcitol in reducing nephrotoxicity of CNIs. Although our study was not designed to assess the protective role of paricalcitol in CNIs nephrotoxicity our data could be considered positive. Indeed, in the follow-up, the stability of renal function was associated with a slight reduction of proteinuria in proteinuric patients and not the appearance of proteinuria in proteinuria-free patients. Even though our study lasted only 24 months, in RTRs treated with cyclosporine we did not notice any changes in renal function and proteinuria that remained stable throughout the study. In RTRs treated with cyclosporine during the study, there were no changes of blood pressure values, of proteinuria, and of the number of patients treated with renin-angiotensin blockers or their dosage. On the contrary in RTRs not treated with cyclosporine, there was a reduction of proteinuria, but not statistically significant. Finally, it is not well known whether paricalcitol may interfere with the metabolism of CNIs influencing their serum levels. In our experience, we did not record any change in the serum levels of CNIs which lead to the need for adjustment of their doses.
Our study has some limitations represented by a low number of the patient’s sample; lack of a control group; lack of an accurate measurement of the glomerular filtration rate. However, these limitations are partially offset by the fact that the patients were evaluated prospectively, and with an adequate duration of the study. The duration of the observation allowed us to state that paricalcitol is a well-tolerated and an effective drug and moreover does not seem to affect renal function. Furthermore, in our study, there was frequent monitoring of various laboratory parameters, that are not always evaluated in other studies.
In conclusion, long-term treatment of SHPT after renal transplantation with low doses of paricalcitol was effective in reducing PTH levels with very few episodes of hypercalciuria and/or hypercalcemia not resulting in the definitive suspension of therapy. Renal function was stable throughout the study period, even in those patients who were taking cyclosporine. This finding suggests that we can not rule out a nephroprotective role of paricalcitol in patients at high risk of nephrotoxicity. All this makes the paricalcitol therapy effective and well tolerated.
Download Provisional PDF Here
Article Type: Research Article
Citation: Massimetti C, Imperato G, Feriozzi S (2017) Effectiveness and Safety of Oral Paricalcitol Therapy on Long-Term Treatment of Normocalcemic Persistent Secondary Hyperparathyroidism in Renal Transplant Patients. Transplant Res J 2(2): doi http://dx.doi.org/10.16966/2473-1730.111
Copyright: © 2017 Massimetti C, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Publication history:
All Sci Forschen Journals are Open Access