Table 1: Composition of Somah Organ Preservation solution (pH 7.5)

Haiyan Cao1,2,3# Samar K Lowaleka1,2,3# Arun Chaudhury1,2,3 Xiu-Gui Lu1,2,3 Patrick R Treanor3 Hemant S Thatte*1,2,3
1Cardiothoracic Surgery Division, Department of Surgery, Harvard Medical School, USA*Corresponding author: Hemant S Thatte, Department of Surgery, VA Boston Healthcare System 1400 V. F. W. Parkway, West Roxbury, MA 02132,USA, Tel: 857-203-5919; Fax: 857-203-5592; E-mail: Hemant_Thatte@hms.harvard.edu
Background: Paucity of available organs is a major issue in liver transplantation. Even though DCD (donation after cardiocirculatory death) livers can be a source, such livers are rarely used because of potential ischemic injury that can lead to graft failure. However, if preservation conditions can be optimized for DCD liver storage that prevent injury, facilitate recovery and preserve function, thereby facilitating increased high quality organ supply. This pilot study was designed to evaluate our novel storage solution Somah in its ability to maintain phosphate synthesis of livers and halt progression of long-term static storage-dependent multicellular damage.
Methods: Different biochemical and histomorphological parameters of porcine DCD livers stored in Somah solutions at 4°C were examined at 0, 1, 6, 24 and 72 hours and compared with the currently used standard University of Wisconsin solution (UWS).
Results: In UWS stored livers, time-dependent attenuation of total phosphates and hepatocellular damage, revealed by histopathology and enzyme release, suggested marked loss of tissue viability. In contrast, hepatocytes, endothelial cells and bile ductular epithelia appeared intact in Somah-preserved livers. Metabolism to maintain ex- vivo phosphate synthesis was maintained and extracellular liver enzymes release was low in comparison. Increase in albumin synthesis was apparent upon reperfusion of Somah-preserved livers.
Conclusion: Extracorporeal storage of livers in novel Somah solution provides a potential superior avenue to protect integrity of hepatocytes, endothelia and cholangiocytes, maintain microcirculatory patency and sustain liver function during extended organ storage. Implementation of Somah as a storage medium may lead to optimal graft function and may impact outcomes favorably after hepatic transplantation, a subject of future investigation.
Hepatocytes; Endothelium; Cholangiocytes; Preservation Solution; Transplantation
Despite advances in transplant surgery, post-operative outcomes after solid organ transplantations have shown only moderate improvements [1]. This is related to inclusion of organs from marginal DCD (Donation after Cardiocirculatory Death) donors instead of BHD (beating heart) donors [2-4]. In spite of procedural morbidity, liver transplantation remains the treatment of choice for end stage liver failure, which otherwise culminates in fatal outcomes [3]. Maintaining organ viability during extracorporeal storage prior to transplantation is an important prerequisite for successful post-transplant long-term outcomes. This is dependent on status of the harvested organ and temporal explant storage environment. Optimal preservation conditions for DCD livers could thus increase high quality organ supply.
In most transplant centers, livers are cold flushed and simple cold stored, preferably forless than 12 hours prior to transplantation. However, investigators are heading towards perfused storage at hypothermic to normothermic temperatures in an effort to improve quality of organs for transplant [4,5]. While loss of high energy phosphates (HEP), endothelial dysfunction, microcirculatory blockade and collapse of bile ductular function are the most significant parameters that pertain to short and long-term graft malfunctions because of which DCD livers are rarely used [6-15], successful functional transplant rates in current practice fall significantly beyond 12 hour storage [16]. This is due to initiation of multifactorial degenerative cascades mainly resulting from progressive loss of HEP during hypoxia [17].
Somah, an organ storage solution of unique composition, rationally designed based on principles of biochemical synergism [18] (Table1), provides various energy substrates, metabolic modulators, free radical scavengers and antioxidants, ammonia chelators, nitric oxide synthase substrates and intracellular and extracellular H+ modulators. We hypothesized that these components would prevent edema, ionic imbalance, and energy depletion and provide cellular support during ex vivo storage of solid organs [18-23]. Recently completed preclinical studies demonstrate that hearts can be stored at ambient temperature and for extended period in Somah in fully functional state, unlike in the clinical comparators [18,20-23]. We sought to extend the evaluation of Somah in preservation of porcine abdominal DCD organs obtained post extraction of the hearts [21-23]. Based on our preliminary studies in rat DCD abdominal organs demonstrated the structural and biochemical preservation of visceral organs upon prolonged hypothermic storage in Somah [19]. Protection ex vivo of structure and function of cardiomyocytes, endothelium [18,20-23] and hepatocytes [19] observed during short-term and prolonged static storage of BHD and DCD donor hearts, livers and kidneys in Somah corroborate this hypothesis [18-23].
We hypothesized that Somah because of the multifactorial ability to modulate metabolism offers a superior avenue for cytoprotection and recovery in the liver during storage, a highly vascular organ of heterogeneous cell populations. The aim of this current pilot study was to evaluate the comparative efficacy of Somah with the currently clinically used University of Wisconsin solution (UWS) in their ability to preserve and potentiate recovery of DCD porcine livers in vitro during a 72 hour period of hypothermic storage. We also performed limited extracorporeal hepatic reperfusion and functional evaluation of Somah-stored livers to test the feasibility for future transplant studies to evaluate graft function.
CoStorSol (UWS) (Preservation Solutions Inc, Elkhorn, WI) and Somah (Somahlution, LLC, Jupiter, Fl), Table 1, was compared for storage properties. All other chemicals were obtained from Sigma-Aldrich (St. Louis, MO). VetScan iStat, VetScan VS2, CG4+, CG8+, Large Animal Profile, Comprehensive Diagnostic Profile cartridges for measuring blood gases, electrolytes, lactate, glucose, aspartate transaminase (AST), alanine transaminase (ALT) and creatine kinase (CK) enzymes were purchased from Abaxis Inc (Union City, CA).
Liver storage and procurement of samples: The study was conducted in fourteen female swines, each weighing 40-50 Kg in accordance with protocol approved by our Animal Studies Subcommittee (IACUC), VA Boston Healthcare System. The animals were divided into two groups of seven animals each. Whole livers were dissected out 60 ± 10 minutes after cardiac death and extraction of heart [21-23]. The livers were stored in UWS (UWS livers) or Somah solution (Somah livers) for 72 hours at 4°C. The solutions were not replaced during storage. Liver biopsies were obtained at 0, 6, 24 and 72 hours for imaging and biochemical assessment of viability. UWS and Somah solutions were sampled at 1, 6, 24 and 72 hours for metabolic monitoring and for other assays and compounds relevant to liver function.

Table 1: Composition of Somah Organ Preservation solution (pH 7.5)
General anesthesia was induced with i.m. injections of telazol 4-6 mg/ kg and xylazine 2 mg/kg. After intubation, animals were maintained with i.v. propofol (10 mg/kg/hour), remifentanyl (40-60 µg/hr) and nimbex (cisatracurium) 10-20 mg, and mechanically ventilated. Aorta was crossclamped, heart arrested, and heart-lung block was extracted for other experiments as described [20-23]. After median laparotomy, suprahepatic aorta was cannulated, and abdominal organs flushed with 2 L of ice cold UWS or Somah solution at a pressure and flow rate of 100 mmHg and 300 ml/min respectively, till the perfusate returning through the suprahepatic inferior vena cava (IVC) was clear. The harvest was concluded with a total hepatectomy. Livers were transferred to plastic bags containing preservative solutions maintained at 4ºC in an icebox, and transported within 30minutes to the lab for further analysis. Livers were transferred to storage box containing preservative solutions and stored at 4ºC for another 72 hours.
ATP and creatine phosphate (CP) were measured in liver tissue extracts as described [19]. In brief, 20 mg of hepatic tissue was suspended in 400 µl of 0.4 M ice-cold perchloric acid and homogenized twice for 30sec. Homogenate was centrifuged at 1970 g for 10mins at 0°C. An aliquot of supernatant was neutralized with equal volume of ice-cold 0.4 M KHCO3 solution and centrifuged as mentioned above. The supernatant was stored at -80°C for ATP and CP measurements. The pellet was dissolved in equal volume of 0.1 M NaOH and centrifuged and used for protein assay. ATP and CP were measured using a bioluminescent assay kit (Sigma- Aldrich and GloMax-Multi+ Detection System, Promega) according to the protocol provided by manufacturer.
Preparation of blood for Ex vivo studies: Systemically heparinized blood was collected intraoperatively, leukodepleted and stored at 4°C. Prior to the commencement of experiments, the hematocrit was adjusted to 20% using Somah solution (now perfusate). The perfusate, pH, glucose, K+, Ca2+ and HCO3 - were adjusted for swine blood levels (7.5; 100 mg/dl; 3.7, 1.38, and 32 mmol/l respectively), using 10% dextrose, KCl, CaCl2 and NaHCO3 , respectively; gases were adjusted as required.
Ex vivo perfusion: Livers were stored in Somah for 72 hours at 4°C (n=3). Hepatic artery and portal vein were identified and cannulated. The livers were kept in a polypropylene perfusion chamber attached to our custom built Somah Device that we use for ex vivo reanimation of hearts [20-23]. An oxygenator, heat exchanger, clinical documentation improvement (CDI) monitor and data acquisition device with custom written software (Comdel Inc, Wahpeton, ND) were incorporated into the system for real-time monitoring of changes in perfusate pH, temperature, pO2 , pCO2 , K+ and HCO3 -apop, pressure and flow rates. Somah device reservoir was filled with 2 L perfusate. Livers were gently flushed through the portal vein with 2 L of cold Somah, and then connected to Somah device via the hepatic artery (HA) and portal vein (PV). The reservoir outlet was diverted into two circuits: in the first circuit, the perfusate drained by gravity into the PV at a pressure of 8-10 mmHg (adjusted by changing the height of the reservoir). In the second circuit, perfusate was diverted through a pump to the HA (at pressures of 80-100 mmHg) via the oxygenator and heat exchanger. Temperature of perfusate was raised to 37°C over a 20 min period and the perfusate was circulated through the liver for next 2 hours. The liver perfusate drained into a chamber through hepatic veins (HV) and was returned to the reservoir by another pump. Perfusate draining from the HV was temporally sampled for albumin, liver enzymes and other metabolites as mentioned below. Because of damage to the DCD livers stored in UWS, this functional assessment was performed only in Somah-livers.
Analysis of metabolites and liver enzymes: Blood parameters were assessed in perfusate inflow (HA) and outflow (HV). CDI monitor, VetScan iStat and VetScan VS2 were employed to determine biochemical parameters, blood gas, albumin synthesis and liver enzymes including alkaline phosphatase (ALP), Alanine aminotransferase (ALT), Aspartate aminotransferase (AST), γ-glutamyl transpepstidase (GGT) and creatine kinase (CK).
Histopathology: Tissue biopsies of livers stored either in Somah or UWS were taken at 0, 6, 24 and 72 hour storage time points, fixed in 10% formalin and processed for histopathology (10 µ sections; Hematoxylin and Eosin stain). The images were acquired and analyzed using Olympus microscope and image analyzer system (BX51TRF; Olympus America Inc, USA). Images were assessed blindly for histopathology by three independent observers.
Statistical analyses: The measurements and data extraction were performed in a blinded fashion. Comparison within the two groups (UWS vs. Somah, n=7 in each group; static storage) was conducted to evaluate the effect of the two solutions on organ function. The quantified initial values of various assays were compared to subsequent time points within each group using one-way analysis-of-variance (ANOVA), followed by Dunnett’s multiple comparison test and t-test for comparative analysis between paired groups. Statistical significance was accepted at 95% confidence level (P<0.05). All values used were mean ± SEM unless otherwise indicated. All analyses were performed using GraphPad Prism 6 (version 6.1), using a 0.05 significance level.
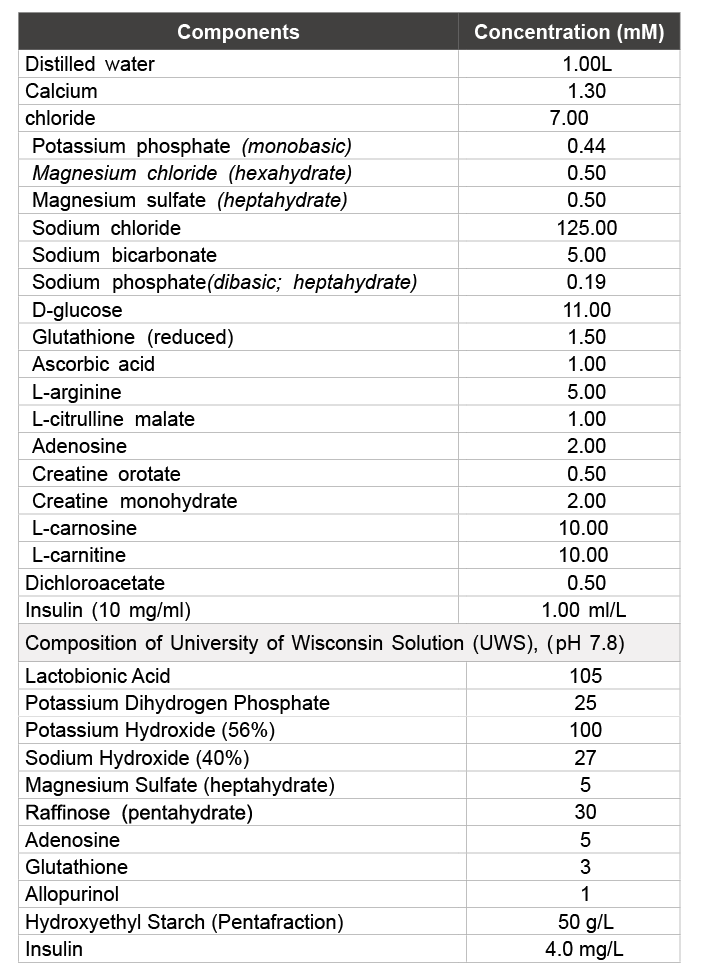
Gross appearance: Upon storage in UWS at 4°C, the gross appearance of livers was rapidly altered to a discolored state within the first hour of storage and subsequently continued to temporally deteriorate (not shown). In contrast, livers stored in Somah maintained their color similar to freshly extracted livers (control), even after extended 72 hour storage at 4°C (Figure 1). Furthermore, there was no weight gain by the Somah livers during storage (not shown).
Hepatocytes: Nuclear chromatin condensation and pyknotic changes of hepatocytes in numerous low power fields were seen in livers stored in UWS but not in Somah. Binucleate and polyploid hepatocytic nuclei were consistently seen in sections obtained from both UWS and Somahpreserved livers. Furthermore, in Somah livers, cellular boundaries between adjacent hepatocytes were visibly intact, and no cholestasis or bile canalicular dilatations were apparent upon careful scan of multiple fields. In sections obtained from UWS livers at 72 hrs, degeneration of hepatocytes associated with extensive vacuolation was evident, but not in Somah livers (Figure 2).
Biliary ducts and ductules: Nuclei lining both bile ductules and larger bile ducts in the portal triad appeared intensely pyknotic, suggesting apoptosis or necrosis of cholangiocytes in UWS livers. In contrast, the bile ducts and ductules were uniform in appearance with regularly placed nuclei and well preserved nuclear heterogeneity in Somah livers after 72 hrs of storage (Figure 2). Furthermore, in UWS livers, there was denudation of bile ductular epithelium and sloughing of the mucosa along with disorganization of the basally placed nuclei of the ductules as early as 6 hr time point (Figure 3). In contrast, such denudation of bile ductular epithelium was not visible in Somah livers even after 72 hours of storage (Figures 2 and 3).
While the starting pH for freshly reconstituted UWS and Somah was alkaline (UWS pH being higher than Somah), it was more acidic (6.8- 7.0) in Somah compared to UWS (7.3-7.8) during all storage time points. There was a greater increase in lactate in UWS despite a lesser decrease in pH. Production of lactate did not correspond with sharp drop in solution pH, perhaps due to high buffering capacity of both the solutions. There was 1.5-fold increase in lactate levels in the UWS in comparison to Somah solution, which was significantly different at 24 and 72 hours, respectively (p<0.05) (Figures 4A and 4B).
As shown in Figure 4 (lower panel), there was a comparable increase in glucose concentration in both UWS and Somah solution. Glucose concentration in UWS increased from 0 mg/dl to as high as 140 mg/ dl at the end of 72 hour storage. Similarly, the glucose levels in Somah increased by 1.72 fold to 320 mg/dl over baseline levels of 180 mg/dl during the same time period, indicating greater glycogen breakdown than glucose reuptake.
Oxidative phosphorylation is attenuated by hypothermia. Since in an open system as used in our experiments, the solubility of atmospheric oxygen in water is inversely related to temperature, both Somah and UWS were supersaturated with oxygen at 4°C, with a pO2 of 200 ± 13 mmHg at the start of storage. Therefore, utilization of oxygen by the livers during extended temporal hypothermic storage cannot be clearly demonstrated, as oxygen consumption, if any, follows zero order kinetics.
On the contrary, while the pCO2 , an indicator of dissolved CO2 , does not change in Somah or UWS solution in the absence of organs (not shown), our studies demonstrated a significant increase in pCO2 in Somah with stored liver during the 72 hour storage period, indicating oxidative metabolic turnover in Somah-stored DCD livers. In contrast, CO2 levels were significantly lower and remained unaltered in UWS with stored liver during the 72 hour period. In UWS, pCO2 measured at 30 minutes (6.30 ± 0.38 mmHg) after the immersion of the liver and transfer to the lab increased insignificantly to only 7.33 ± 0.48 mmHg over 24 hours and 9.27 ± 0.89 mmHg at 72 hours storage. In contrast in Somah, the pCO2 increased significantly from 10.8 ± 1.13 mmHg within 30minutes after initial immersion of the liver to 15 ± 1.45 mm Hg at 1hour, to 27 ± 1.14 mmHg at the end of 72 hours, indicating temporal increase in metabolism. The greater pCO2 in Somah than UWS at 30 minutes is indicative of active metabolism from the start of storage. Probability of bicarbonates present in Somah contributing to this increase in pCO2 was negated by the fact that HCO3-- concentration remained mostly unaltered (4.23 mM/L) during the 72 hour storage. Conversely, HCO3- concentration decreased from 7.30 to 5.33 mM/L in UWS during the 72 hour storage, which may have contributed to the non-significant increase in pCO2 that we observed in UWS during the storage period (Figure 5). Another possibility to be considered is that anaerobic glycolysis, through lactate production, may have contributed to the rise of pCO2 in Somah (as well as the mild rise in UWS stored livers). These data indicate that at least cellular metabolism was kept intact. Further studies are needed to delineate the specific role of oxidative metabolism during ex -vivo storage.
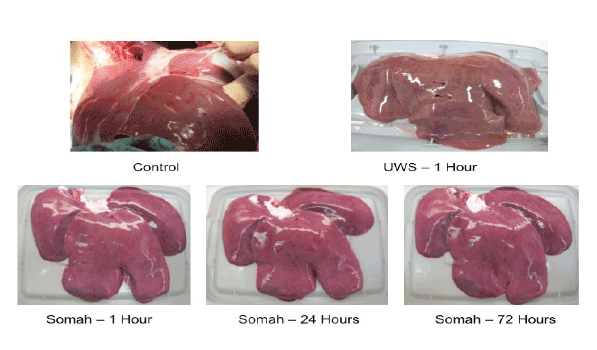
Figure 1: Gross appearance of livers stored in UWS or Somah solutions. Morphology of DCD livers. Liver stored in UWS showed significant discoloration within 1 hour of storage. In contrast, livers stored in Somah maintained their color and morphology throughout the 72-hour storage period. All livers were progressively biopsied for further analysis. Representative images: UWS n = 7; Somah n = 6
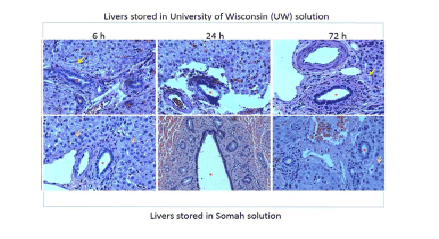
Figure 2: Histopathology of livers at 6, 24 and 72 hrs stored in University of Wisconsin (UWS) and Somah solutions. Note that bile ductules show mucosal ulceration and disorganized, heaped, condensed nuclei in livers stored in UWS. These changes were seen as early as 6 hrs. In contrast, livers stored in Somah for 72 hrs showed normal appearing bile ductules in the portal region with clear, rounded uniform lumen and intact mucosa with regular basal nuclei. Also note that in the upper panels, some periportal hepatocytes show ballooning degeneration and apoptotic nuclei (yellow arrows), while periportal hepatocytes showed normal cellular boundaries and heterochromatic, open-faced nuclei with nucleoli in livers stored in Somah (orange arrows). Red asterisks indicate bile ducts and ductules of different calibers (all images, x200).
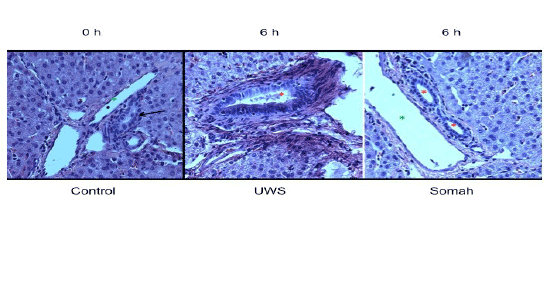
Figure 3: High power view (x400) of bile ductules obtained from livers cold-stored in UWS and Somah at 6 hrs. Note the regularly arranged basal nuclei and clear lumen in a medium sized bile ductule seen at 0 hr (arrow, left panel). In contrast, note the polychromatic appearance of the ductular nuclei, including some condensed nuclei and reactive (proliferative) nuclear changes in the 3 o’clock position of liver stored in UWS. Note the sloughed material obstructing the ductular lumen and non-uniformly stained and ragged appearance of the mucosa. In contrast, lumina of bile ductules were regular appearing with intact mucosa in Somah stored livers. These changes were uniformly seen in bile ducts of different diameters (red asterisks). Green asterisks indicate portal veins/venules (x400).
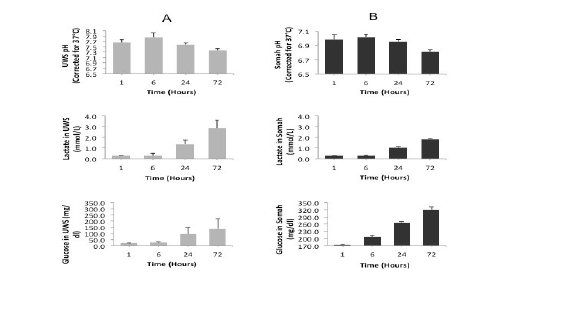
Figure 4: Changes in pH, lactate and glucose levels in livers during storage. Graphs show time-dependent alterations in pH (upper panel), lactate (middle panel) and glucose (lower panel) levels in UWS (column A) and Somah (column B) solutions during extracorporeal storage of DCD livers. Metabolic parameters were temporally assessed in the storage solution during extracorporeal storage of DCD livers in UWS and Somah for 72 hours at 4º C.
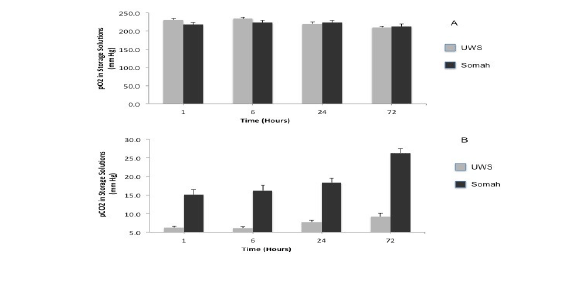
Figure 5: Oxygen consumption and CO2 production in Stored Livers. Graphs show the extent of oxygen consumption (A) and CO2 production (B) during extracorporeal storage of livers in UWS and Somah solutions at 0, 6, 24 and 72 hour time points. *Significant change from baseline levels in Somah
ATP, CP and total phosphate concentrations decreased significantly (p<0.05) in UWS livers during the 72hour storage (Figure 6). The total phosphate decreased temporally in UWS livers during storage, resulting in 32% drop in the first 6 hours, followed by a 50% decrease by the end of 72 hours. In contrast, ATP, CP and total phosphate levels did not change appreciably in Somah livers during the entire course of preservation. We observed an increase in total phosphate concentration at 72 hours in Somah livers (Figure 6), analogous with metabolic production of CO2 .
We measured time dependent release of ALT, AST and CK enzymes in storage solutions as markers of liver tissue injury. Release of all three enzymes was significantly elevated (P<0.05) when livers were stored in UWS, in comparison to livers stored in Somah (Figure 7).
Somah stored livers (72 hours) were reperfused with blood (perfusate) for 2hours at 37°C for functional evaluation. Oxygen consumption by the reperfused livers increased significantly by 38% and 64% (p<0.05) at 30 and 120 min, respectively. Concomitantly, lactate concentration released in the perfusate decreased by 11% and 41% at 30 min and at the end of 2 hours, indicating anaerobic to aerobic metabolic switch.
There was no significant difference in temporal release of liver enzymes during reperfusion (Figure 8). Both ALP (15.33 ± 0.96) and γ-GT (17.5 ± 1.06 U/L) were within physiological concentrations at the end of 2 hours. However, AST (2151 ± 81), ALT (228 ± 32) and CK (1428 ± 205 U/L) concentrations were elevated in the perfusate at the end of reperfusion. There was a significant increase in synthesis and release of albumin by the Somah livers within 30min after reperfusion (p<0.03), which increased temporally over the 2hour period (p<0.01) (Figure 9). Synthesis and release of bile was also increased temporally in perfused Somah livers (data not shown).
The objective of this pilot study was to compare “Somah” and UWS solution for extended temporal extracorporeal preservation of porcine DCD livers. In this study, we provide evidence of efficient storage properties with of Somah over UWS to functionally salvage DCD livers. We hypothesized that the unique formulation of Somah temporally maintains and/or augments energy state of an organ during extracorporeal storage, resulting in enhanced cellular homeostasis and structural integrity, thereby, effectively improving the overall repair and recovery of excised organ during the storage period.
The results of the present study explicitly reveal that frank progressive histological injury of DCD livers are potentially preventable, depending upon the composition of storage solution. The karyopyknosis in hepatocytes and reactive changes of biliary epithelial cells were visible as early as 6 hrs in UWS-livers, but not in Somah-livers at 72 hour time point. Furthermore, this study provides detailed findings of different kinds of biliary injury, earlier only scantily reported [12,15,20,21]. DCD livers stored in UWS showed degenerative changes in both small and large bile ducts, a potential cause of non-anastomotic biliary stricture and biliary dysfunction.
This has been reported to result in poor post-transplant graft functioning [12] and greater morbidity [14]. Possibly, the higher K+ levels and ischemia may have augmented cellular damage in UWS-livers [22]. Furthermore, while acidic pH is reported to be constitutively beneficial to hepatocytes and sinusoidal epithelial cells [23], the pH of Somah, compared to UWS remained relatively acidic throughout the preservation period. The enzyme assay studies showed significantly diminished hepatocellular injury of livers stored in Somah in contrast to UWS.
While evidence of glycogenolysis (indicated by increased glucose levels) and glycolysis (indicated by increased lactate levels) was present in both UWS and Somah stored livers, the oxidative metabolism of glucose probably occurred in Somah-stored livers. With sufficient O2 to meet the metabolic needs of an explanted liver, the process of respiration may be regulated such that ATP is resynthesized as rapidly as it is utilized [24]. Mitochondria need only minute amounts of oxygen (0.05 mmHg; 10-7 mol/L) for an operational oxidative phosphorylation [25-27] which is not entirely attenuated at hypothermic storage [28].
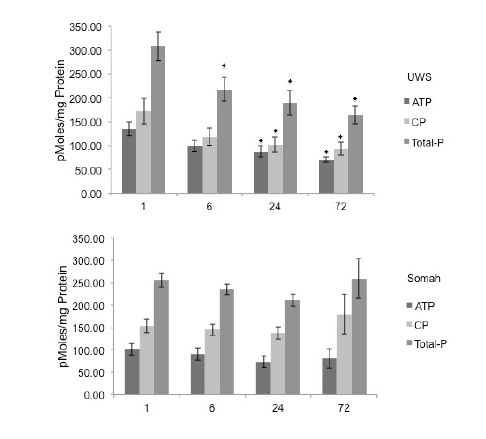
Figure 6: Total phosphates in Stored Livers. Graphs show time-dependent changes in ATP, CP and total phosphate levels in liver tissue during long-term extracorporeal storage of DCD livers in UWS (upper) and Somah (lower). *p <0.05 , Compared with 1 Hour.
While during hypothermic perfused organ storage, glycolysis appears to be the primary energy source at a pO2 of 150 mmHg [29], the dichloroacetate (DCA) in Somah likely diverts the pyruvate generated by glycolysis into Krebs cycle, thus further enhancing ATP synthesis and maintenance of phosphates (Figure 6). Furthermore, the DCA, by enhancing oxidative metabolism of pyruvate, also prevents build-up of lactate in Somah stored livers. Moreover, insulin, which enhances the entry of glucose into cells, is a hepatotrophic factor and is essential for maintenance of hepatic ultrastructure and regenerative ability [30]. Somah solution exploits this ability of insulin by providing it in a concentration of 100 U/L, 2.5 folds higher than that in UWS. Thus, the greater lactate accumulation above threshold levels in the absence of DCA and the lower insulin concentration in UWS-livers are likely to have contributed to the comparative changes in this group [31].
However, we extend caution in our observations that the higher pCO2 in Somah stored livers may also have resulted from sustained anerobic metabolism. This shows ability to generate energy- providing phosphates even ex vivo, but requires additional experiments to compare the contributions of anerobic versus oxidative metabolism to these processes.
Loss in phosphates in the explanted organs during storage leads to irreversible degenerative changes in the organ [8]. Despite an equivalent increase in glycogenolysis-dependent glucose concentration in both UWS and Somah solution livers during storage, there was a depletion of phosphate stores in UWS, in contrast to enhancement in Somah. This suggests that UWS livers are in fact in a catabolic state, resulting in loss of phosphates. In contrast, by promoting oxidative phosphorylation of glucose in Somah-livers, a 15 folds greater amount of phosphates are generated (for equivalent glucose molecules) than by anaerobic glycolysis alone [32]. Furthermore, during hypothermic perfusion, livers having greater ATP levels demonstrate lower oxidative stress upon rewarming [33].
Static organ preservation has always been recognized as critical component in maintaining liver explants prior to transplantation [34]. Limited studies have addressed the deleterious effect of storage of livers in UWS solution [35, 36]. However, this does not appear to occur in DCD livers preserved in Somah. Preliminary functional studies indicate that there is a rapid switchover to aerobic from anaerobic metabolism, supported by similar observations in the heart [21-23]. Similarly, there was a significant increase in synthesis of albumin, and release of bile in the reperfused Somah livers, indicating that metabolism and function are preserved after prolonged storage. We did not evaluate UWS-stored livers for functional recovery because of gross damage to these organs.
The biliary system in Somah livers remains patent and intact, demonstrating lack of frank damage. It is well known that hepatocytes can repair or regenerate, especially if the energy state of the organ can be preserved. It is the failure of biliary system that leads to PNF and DGF [12]. Biliary dysfunction may be prevented by ex-vivo storage in Somah, which may be tested by transplantation studies.
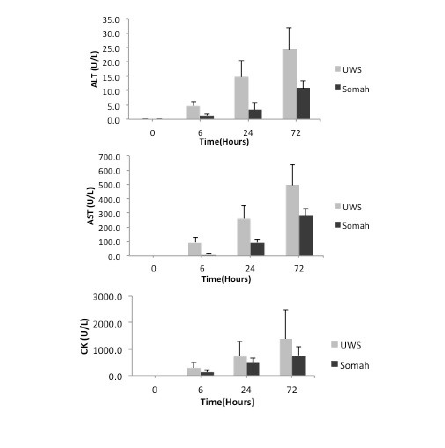
Figure 7: Release of liver enzymes during organ storage. Release of liver enzymes during extra- corporeal preservation of DCD livers was determined in the respective UWS or Somah solutions at time 0, 6, 24 and 72 hours. ALT (upper), AST (middle) and CK (lower) levels were evaluated.
In conclusion, the results of the present study demonstrate that Somah has the ability to preserve biochemical and physiological function like albumin and bile secretion capability in DCD livers ex vivo. While the present investigation provides foundational evidence for possible prolonged extracorporeal storage of DCD livers in Somah, conduct of actual liver transplants for both BHD and DCD donor livers is the necessary next step to elucidate the comparative efficiency of the storage solutions ex vivo.
We gratefully acknowledge the assistance of Diane Ghera, BS and her staff with animal husbandry and preparation of animals for surgery. We thank Peter Hirsch, MS for his assistance during the animal lab pre and post surgery. We would like to thank Aditi Thatte for her encouragement and Dr. Frances Achee and Michael Charpak for administrative support.
Supported by Merit Grant (HST), Department of Veterans Affairs, and an unrestricted gift from Somahlution (Jupiter, Fl) to Boston VA Research Institute for research (HST)
Author Disclosure: None of the authors have any conflict of interest that could affect the design, execution, interpretation, writing and publication of this research.
HC – Assisted in Surgery, conducted experiments, analyzed data, wrote manuscript SKL–conducted experiments, assisted in surgery, analyzed data and writing of manuscript AC – conducted imaging and histopathological evaluation; wrote manuscript
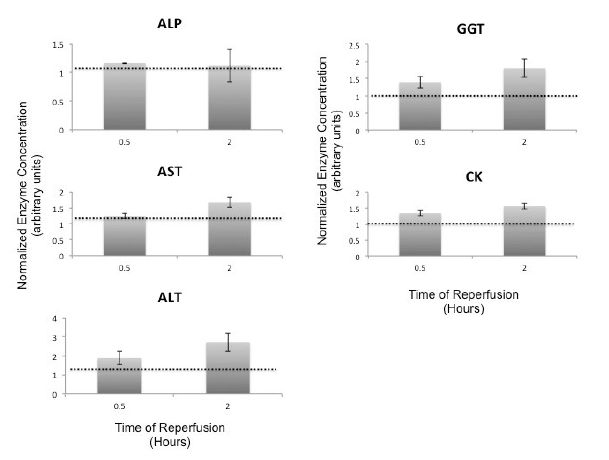
Figure 8: Reperfusion Induced Release of Liver Enzymes. Release of liver enzymes during extra- corporeal reperfusion of DCD Somah livers was determined in the perfusate (HV) at time 0 (single pass), 0.5 and 2 hours. ALP, GGT, AST, ALT and CK levels were evaluated. Due to intra variability of enzymes in 72-hour stored blood in the reconstituted perfusate, data was normalized to time 0 hour values; mean ± SEM, from independent experiments.
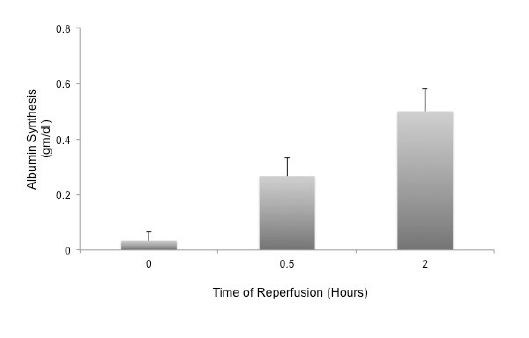
Figure 9: Reperfusion Induced Synthesis and Release of Albumin by Livers stored in Somah for 72-hours. Somah livers temporally synthesized and released albumin in the perfusate (HV). Increase in albumin synthesis was highly significant at 0.5 hour (P<0.03) and at 2 hours (P<0.01). Values represent mean ± SEM from independent experiments.
XL – conducted blood analysis during surgery and biochemical analysis of Somah
PRT – assisted as perfusionist during and post surgery in the lab; anesthesia, surgery, cardioplegia HST – obtained funding, designed experiments, performed surgery, helped analyze data and writing of the manuscript; final approval of the manuscript
Download Provisional PDF Here
Article Type: Original Article
Citation: Cao H, Lowalekar SK, Chaudhury A, Lu XG, Treanor PR, et al. (2016) In vitro Evaluation of Porcine Livers Stored in Novel Solution Somah. Transplant Res J 1(1): doi http://dx.doi. org/10.16966/trj.101
Copyright: © 2016 Cao H, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Publication history:
All Sci Forschen Journals are Open Access