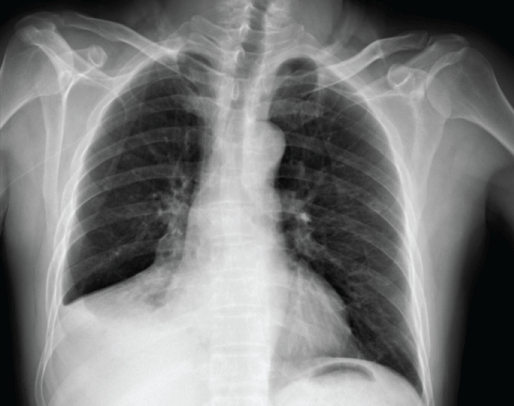
Figure 1: Chest radiograph at hospitalization showed a lung mass in the right middle-lower field.
Xia Ning1 Haiyan Xie1 Zejun Zhou1 Jue Zou2*
1Department of Respiratory Medicine, Nanjing Brain Hospital (Nanjing Chest Hospital), Nangjing, China*Corresponding author: Jue Zou, Department of Pathology, Nanjing Brain Hospital (Nanjing Chest Hospital), No. 215 Guangzhou Road, Gulou District, Nangjing, 210000, China, E-mail: zoujue1981@126.com
A 56-year-old Asian male with 20 years smoking history was admitted to the hospital due to a cough with sputum production. Chest Computed Tomography (CT) revealed a tumor shadow in the right middle lung, and transbronchial biopsy diagnosed Lung Squamous Cell Carcinoma (LCSS). Further molecular pathological assessment detected Epidermal Growth Factor Receptor (EGFR) sensitive mutation, while Programmed Death-Ligand 1 (PD-L1) was highly expressed with a Tumor Proportion Score (TPS) of >70%. Tumor Proportion (TP) regime was the first-line of treatment gained an obvious reduction in tumor size. Until head progression was found, the patient took Osimertinib orally and is currently stable. Given the rarity of the LCSS with EGFR mutation and high-expressed PD-L1, this case report provides a successful clinical treatment option for doctors in similar situations.
Lung squamous cell carcinoma; EGFR mutation; Programmed death ligand 1; TP regimen
Epidermal Growth Factor Receptor (EGFR) mutations are found in around 10-15% of Caucasians and about 50% of Asian patients with Non-Small Cell Lung Cancer (NSCLC). Especially in non-smoking and female Asians, the proportion of EGFR mutation could reach approximately 60.7% and 61.1%, respectively [1]. Epidermal Growth Factor Receptor-Tyrosine Kinase Inhibitors (EGFR-TKIs) has proven to be a powerful anti-tumor mediation as a lung cancer targeted therapy. Most patients that harbor EGFR sensitive mutations could benefit from EGFR-TKIs and gain longer survivals compared with conventional chemotherapy. However, it can reveal in de novo EGFRTKIs resistance in patients with EGFR sensitive mutation and PD-L1 expression [2].
Programmed Death receptor-1 (PD-1) and its (PD-L1) inhibitors also displayed striking and durable clinical responses in NSCLC patients with high PD-L1 expression. However, EGFR-mutant NSCLC is unlikely to express PD-L1 than those EGFR wild types. Besides, many studies suggested that Immune Checkpoint Inhibitors (ICIs) could not show the efficacy power enough to control cancer cell progression in the setting of EGFR-mutant, PD-L1+, nonsquamous cell lung cancer patients [3]. According to the only Lung Squamous Cell Cancer (LSCC) case we found, first-line immunotherapy by Pembrolizumab led to a poor performance status and caused a rapid cancer progression [4].
Unfortunately, the surveys mentioned previously are mainly based on the experience of NSCLC treatment rather than LSCC. The mature treatment experience of LSCC with EGFR mutation and PDL1 expression is rare. No standard remedy is available for this kind of patient and further accumulated exploration is required. In this article, we reported an LSCC patient with EGFR-TKIs sensitizing mutation and high PD-L1 expression. We hoped it could present a successful clinical treatment pathway for EGFR positive and PD-L1- expressed LCSS patients.
A 56-year-old male with a history of 20 years of smoking presented with cough with sputum production in February 2020. Chest Computed Tomography (CT) scan showed pulmonary lesions in the right middle-lower lungs and multiple mediastinal lymph node metastases (Figure 1). Enhanced CT scan revealed a mass in the right hilus pulmonis and central lung cancer. The patient’s Performance Status (PS) score was 1. Due to the high risk of a hilus pulmonis biopsy, the tissue gained from the transbronchial biopsy was taken for histopathological examination. The pathological results indicated squamous cell carcinoma with bilateral lung metastasis (Figure 2). The molecular pathological test was conducted by PCR and showed EGFR mutation (Exon 19: deletion). Programmed death-ligand expression was examined with 22C3 antibodies (DAKO) with a tumor proportion score (TPS) of 70% (Figure 2d). After two cycles of chemotherapy with Paclitaxel (albumin-bound) combined with Cisplatin (TP regimen), the lesion was significantly reduced. After the fifth chemotherapy, the chest CT and head magnetic resonance imaging (MRI) demonstrated tumor progression. Considering that EGFR mutation was detected, Osimertinib combined with head topical radiotherapy was administered as a 2nd-line setting. After a 2-month course of therapy with Osimertinib, the chest CT and brain MRI displayed a shrinking tumor size compared to images obtained one month previously (Figures 3 and 4). The patient was in a stable condition and took Osimertinib for disease control (Figures 3 and 4).

Figure 1: Chest radiograph at hospitalization showed a lung mass in the right middle-lower field.
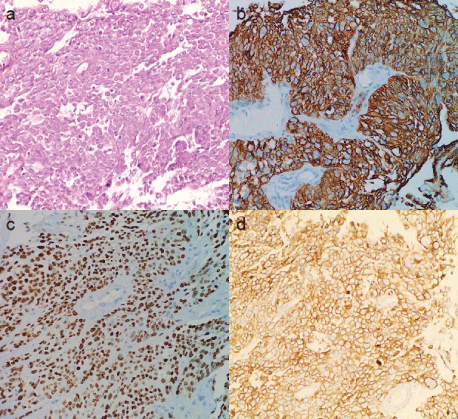
Figure 2: The pathological diagnosis of biopsied tissue was LSCC. (a) Hematoxylin-eosin stain revealed that the right middle lung mass biopsy was consistent for atypical squamous cells, which was partially positive for (b) cytokeratin 5/6 and (c) p40. (d) Furthermore, (PD-L1) showed high expression with (TPS) >70%.
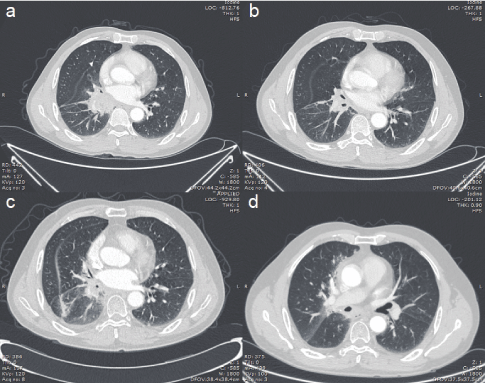
Figure 3: Chest unenhanced (CT) scan: (a) at hospitalization, (b) after 2 cycle TP regimen, (c) after 5 cycle TP regimen, and (d) 1-month Osimertinib therapy.
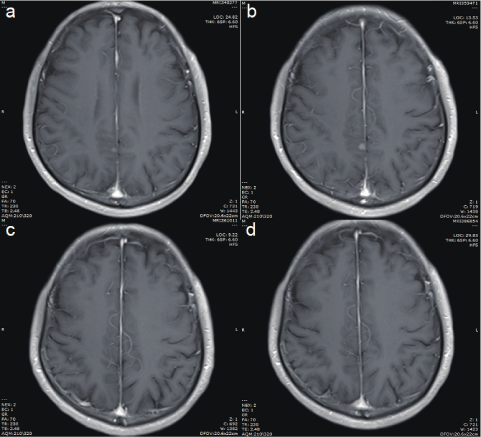
Figure 4: Brain MRI. : (a) at hospitalization, (b) after five cycle TP regimen, (c) after 1-month Osimertinib therapy, and (d) on May 31, 2021.
ICIs revealed a clinically meaningful activity and an acceptable safety profile in nonsquamous cell carcinoma and squamous cell carcinoma. Among patients with advanced LSCC who had been treated previously, immunotherapy would lead to a more significantly meaningful clinical outcome, compared to chemotherapy with Docetaxel [5,6]. For treatment-naive patients, ICI monotherapy could significantly enhance progression-free and overall survival. Moreover, ICI monotherapy was associated with fewer adverse events than chemotherapy, with/ without high expression level of PD-L1 or mutation of driven genes [7- 9]. With respect to the combination therapy, ICI was combined with different types of anti-tumor medicines, like platinumlatinum-doublet chemotherapy [10,11], Iipilimumab [8], and CDK4/6 inhibitor [12], which displayed powerful clinical meanings as a first line Squamous Cell Cancer (SCC) treatment.
There is much evidence that high PD-L1 expression is always associated with longer PFS and OS, especially with a PD-L1 expression level of 50% or more (KY024, IM110). However, EGFRmutant NSCLC patients with high PD-L1 expression could not benefit from ICI-based immunotherapy, according to five trials involving Nivolumab, Pembrolizumab, and Atezolizumab [12,13]. A Phase Ⅱ clinical study was called due to poor efficacy of Embrolizumab [3]. This study enrolled 11 untreated NSCLC patients, of whom 64% had sensitizing EGFR mutations and 73% had PD-L1 expression ≥ 50%. This finding might be the reason why few studies have been done on the immunological treatment of EGFR-mutant and PD-L1 high-expressed NSCLC patients. Not to mention that the reports on LSCC patients with EGFR-mutant and high-expressed PD-L1 were even fewer. The sole LSCC case with EGFR-positive and PD-L1 high-expression showed that 1st-line immunotherapy was not effective and the patient died within six months [4]. Hence, ICIs should be considered only after potential effective therapies have been exhausted.
In our case, chemotherapy with Paclitaxel (albumin-bound) combined with Cisplatin was administrated as the first line treatment owing to the patient’s feasible conditions (PS score=1) and the low EGFR mutation frequency. After head and chest progression was demonstrated five months later, Osimertinib was added to the therapeutic regime, which effectively controlled the tumor size. The one1-year follow-up revealed that the patient obtained an excellent benefit. Instead of first-line ICI treatment, chemotherapy may be taken as the first-line treatment in EGFR-mutant NSCLC with high PD-L1 expression. In cases of brain metastaseis, Osimertinib, considering its good potential in crossing the blood-brain barrier, should be combined in a regime based on imaging and physical conditions. Whether ICI could benefit LSCC patients with EGFR sensitive mutation and PDL1 high expression and the timing when ICI should be administrated requires more treatment experience in these types of patients.
The patients/participants provided their written informed consent to participate in this study.
All data providers have agreed to the publication of this article.
The data that supports the findings of this study are available in the supplementary material of this article.
The authors report no conflict of interest.
This work was supported by Nanjing Medicine Technology Development Funding (No. YKK17185).
JZ participated in study conception and design. XN and XH enrolled and managed patients. XH and ZZ carried out collection and assembly of data. XN, XH and ZZ were involved in data analysis and interpretation. XN and ZZ prepared the manuscript and manuscript figures. JZ edited, critically read, and revised the manuscript. All authors contributed to the article and approved the submitted version.
Not applicable.
Download Provisional PDF Here
Article Type: CASE REPORT
Citation: Ning X, Xie H, Zhou Z, Zou J (2022) Tumor Proportion Regime First-Line Therapy in Metastatic Squamous Cell Lung Cancer with both EGFR Sensitive Mutation and High PD-L1 Expression: A Case Report. J Surg Open Access 8(2): dx.doi.org/10.16966/2470-0991.265
Copyright: © 2022 Ning X, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Publication history:
All Sci Forschen Journals are Open Access