Figure 1: Surface characteristics of the BellaGel® SmoothFine.
Keun Yeong Song1 Jung Youp Sung2 Woo Sik Choi3 Hyung Guhn Lim4 Jae Hong Kim5*
1Department of Breast Surgery, Gwangju Suwan Hospital, Gwangju, Korea*Corresponding author: Jae Hong Kim, Korean Society of Breast Implant Research, The W Clinic, 9F Kukdong B/D, 596 Gangnam-daero, Gangnam-gu, Seoul 06626, Korea, Tel: +82-2-517-7617; E-mail: stenka@hanmail.net
Background: Selection of optimal types of breast implants is an essential factor that determines the degree of patients’ satisfaction with outcomes of augmentation mammaplasty, for which an evidence-based approach to it is mandatory. Several studies have supported the safety of the BellaGel® SmoothFine (HansBiomed Co. Ltd., Seoul, Korea), a silicone gel-filled breast implant from a Korean manufacturer. But such studies have been conducted in a manufacturer-sponsored setting. We therefore conducted this non-manufacturer-sponsored, retrospective study to assess its 3-year safety outcomes in Korean women.
Methods: We performed a retrospective review of medical records in a total of 251 women with a mean age of 31.88 ± 6.83 years old; they received an implant-based augmentation mammaplasty using the BellaGel® SmoothFine. They had been evaluated for incidences of postoperative complications and estimated time-to-events (TTEs).
Results: A total of 48 cases (19.1%) of postoperative complications occurred; these include 22 cases (8.8%) of CC, 8 cases (3.2%) of dissatisfaction with shape, 7 cases (2.8%) of sliding/foreign body sensation, 4 cases (1.6%) of early seroma, 3 cases (1.2%) of early hematoma, 3 cases (1.2%) of infection and 1 case (0.4%) of wound dehiscence. TTEs were estimated at 331.35 ± 12.83 days (95% CI 306.09-356.62).
Conclusions: Here, we describe 3-year treatment outcomes and safety of an implant-based augmentation mammaplasty using the BellaGel® SmoothFine in Korean women. It would be mandatory, however, to perform a meticulous long-term follow-up of patients receiving the BellaGel® SmoothFine and then to consider the possibility that they might be vulnerable to its possible detrimental effects.
Safety; Postoperative complications; Implant capsular contracture; Edema; Seroma
It is of no doubt that assessment of outcomes of an implant-based augmentation mammaplasty from surgeons’ perspectives would be inevitable. On the other hand, however, its aesthetic outcomes and safety profile should also be evaluated from patients’ perspectives. This is not only because they are closely associated with patients’ health-related quality of life, psychosocial and sexual well-being and satisfaction with aesthetic appearance of the breast but also because a patient-oriented evaluation of them has become a critical metric for the quality of treatment in a consumer-driven healthcare environment [1-6].
Selection of optimal types of breast implants is an essential factor that determines the degree of patients’ satisfaction with outcomes of augmentation mammaplasty, for which an evidence-based approach to it is mandatory [7-9].
To date, attempts have been made to preoperatively select breast implants based on anthropometric measurements, such as the width, height and projection of the breast, for the purposes of achieving symmetry of the shape and volume [10-12]. From this context, the BellaGel® (HansBiomed Co. Ltd., Seoul, Korea) is known as a silicone gel-filled breast implant that best fits to Korean women [13]. Its short-term safety has been well described in the literature, which is in agreement with 4- and 6-year interim results of a 10-year prospective cohort study [13-16]. The BellaGel® SmoothFine is the BellaGel® implant with a microtextured surface; it is equipped with softness as well as a refined, smooth surface with a roughness of 5.96 µm, which is a different feature from traditional smooth surface, according to the International Organization for Standardization (ISO) 14607 Annex H Test for surface characteristics (Figure 1). According to the manufacturer, it is advantageous in lowering the rate of capsular contracture (CC) and providing more softness. This might be closely associated with the surface interaction that can decrease macrophage activities and enhance the elasticity of the gel [17].

Figure 1: Surface characteristics of the BellaGel® SmoothFine.
Of note, the safety profile of the BellaGel® SmoothFine has been shown to be non-inferior to that of its competitors [18-20]. But these reports are from manufacturer-sponsored studies.
A recent article revealed that a Korean manufacturer of a silicone gel-filled breast implant, the BellaGel® SmoothFine (HansBiomed Co. Ltd., Seoul, Korea), committed a medical device fraud in violation of the regulatory requirement enforced by the Korean Ministry of Food and Drug Safety (KMFDS) [21]. Interestingly, the BellaGel® SmoothFine has been described as a competitor of the Motiva Ergonomix™ (Establishment Labs Holdings Inc., Alajuela, Costa Rica); its superiority or non-inferiority to other brands of a silicone gel-filled breast implant have been reported in manufacturer-sponsored studies [18-20,22].
The manufacturer’s violation of the regulatory requirement should be considered serious not only because the BellaGel® SmoothFine is one of the most popular brands of a microtextured device in Korea but also because it has been exported to over 30 countries worldwide as a CE-certified device [21].
Given the above background, we conducted this non-manufacturersponsored, retrospective study to assess 3-year safety outcomes of an implant-based augmentation mammaplasty using the BellaGel® SmoothFine in Korean women.
We evaluated the patients receiving an implant-based augmentation mammaplasty at our hospitals between September 26, 2017 and August 31, 2020. We included women aged 22 years or older, those with an adequate amount of tissue for coverage of the breast implant and those with availability of follow-up data. But we excluded women with unilateral or bilateral presence of pre-malignant breast lesions, those with mutations in breast cancer genes 1 or 2 (BRCA1 or BRCA2), those with a past history of taking bilateral mastectomy, those with untreated malignancies, those with a past history of sustaining a radiationinduced damage, those with vascular compromise or impaired wound healing, those with abscess or infection, those with a past history of taking any drugs that may interfere with blood clotting or raise risks of developing postoperative complications, those with underlying medical conditions that may raise risks of developing postoperative complications (e.g., obesity, diabetes mellitus, autoimmune disease, chronic lung, severe cardiovascular disease connective tissue or rheumatoid disease), those who are pregnant or breastfeeding, those with medical conditions that may interfere with wound healing (e.g., active infectious collagen disease, those with active fever (body temperature >38°C), those with severe lung disease (e.g., chronic obstructive pulmonary disease), those with cystic fibrosis, those with active cutaneous or systemic infections, those receiving radiotherapy or chemotherapy within 6 months preoperatively and those lost to follow-up.
We therefore evaluated a total of 287 women (n=287) in the current study; it was conducted in compliance with the relevant ethics guidelines. But informed consent was waived due to its retrospective nature.
We perform an implant-based augmentation mammaplasty in a step-by-step manner, followed by a multi-disciplinary, algorithmbased approach to an early detection of postoperative complications.
Preoperative simulation of postoperative outcomes: Preoperatively, we use the Divina™ 3-dimensional Scanner (Establishment Labs Holdings Inc., Alajuela, Costa Rica) to allow the patients to view possible results of an implant-based augmentation mammaplasty. It not only helps a surgeon obtain anthropometric measurements of the breast, such as breast base width, breast base height, distance from the sternal notch to the nipple, distance from the nipple to the midline, distance from the nipple to the inframammary fold, areolar diameter, internipple distance, intermammary distance and breast volume, but also visualizes its preoperative characteristics. Thus, it stimulates possible results through an analysis of data and information about diverse types of a silicone gel-filled breast implant for the purposes of helping patient select optimal types of a breast implant and thereby yielding satisfactory outcomes (Figure 2).
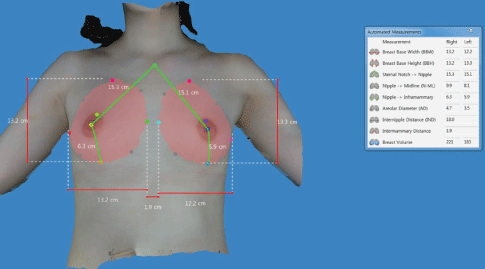
Figure 2: Preoperative simulation of postoperative outcomes using the Divina™ 3-D Scanner.
Anthropometric measurements are preoperatively obtained; these include breast base width, breast base height, distance from the sternal notch to the nipple, distance from the nipple to the midline, distance from the nipple to the inframammary fold and breast volume.
Surgical procedures: Our surgical procedures are performed in compliance with the American Society of Plastic Surgery (ASPS) recommendations.
Peri-areolar, inframammary fold (IMF) and trans-axillary incisions were made under general anesthesia and intravenous sedation for the purposes of preventing visible scarring. Selection of surgical incision is based on our desired outcomes, types of breast implants, the degree of augmentation, the anatomical characteristics of patients and patientsurgeon preference. Based on the Ranquist formula, we determined the distance extending from the nipple to the IMF, the size of breast implant and the scope of dissection. After the dissection, each breast was irrigated using a 100 cc of normal saline mixed with H2 O2 solution at a ratio of 1:1, followed by the use of betadine 100 cc. Then, a breast implant was immersed in a normal saline mixed with ceftezole 1 vial and gentamycin 1 ample and then inserted in a pocket either under the pectoralis muscle (a submuscular placement) or in the retromammary space above the pectoralis major muscle (a subglandular/submammary placement). Methods for inserting and positioning a breast implant in the pocket were dependent on its types, the degree of augmentation, characteristics of a patient’s body and our recommendations. Thus, we performed a dual-plane I/II augmentation on a case-by-case basis. Intraoperatively, the patients were intravenously given ceftezole 1.0 gr. Incisions were closed using layered sutures in the breast tissue. In addition, skin adhesive or surgical tape was used to close the skin [13,23].
Sonographic measurement of capsule thickness: To make an accurate diagnosis of CC, we measure the capsule thickness at 3 months postoperatively in the patients who are suspected of having CC (Figure 3). Moreover, we consider an empirical correlation between the Baker grading system and the capsule thickness on breast ultrasound (Table 1). If necessary, we perform capsulectomy and thereby collect tissue samples to make an accurate diagnosis of complications (Figure 4).

Figure 3: Sonographic measurement of capsule thickness.
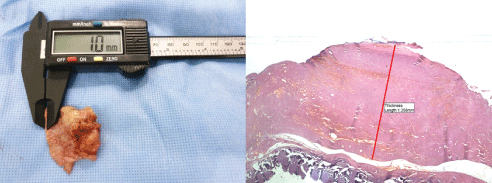
Figure 4: Microscopic measurement of capsule thickness on tissue samples.
(A) The thickness of the entire capsule is measured using an electronic ruler, which is followed by comparison of it with that visualized on breast ultrasound. Thus, almost lack of a difference in the capsule thickness between the two methods is confirmed. (B) A histopathologic examination is performed a stereomicroscope (Olympus SZ61; Olympus Optical Co., Tokyo, Japan), and its findings are photographed using the TUCSEN H series digital camera (Fuzhou Tucsen Photonics Co., Fuzhou, Fujian, China) (hematoxylin & eosin, 40x).
| Baker grade | The capsule thickness on breast ultrasound |
| I | <0.4 mm |
| II | 0.4-0.8 mm |
| III | 0.8-1.4 mm |
| IV | >1.4 mm |
Table 1: Correlation between the Baker grading system and the capsule thickness on breast ultrasound.
Planning of revision surgery based on sonographic findings: We immediately plan for revision surgery considering the capsule thickness and whether the patients present with any notable signs and symptoms when they had an increase in it on breast ultrasound at 3 months postoperatively. If necessary, we frequently perform a followup of the corresponding patients to examine whether they present with changes in the capsule thickness and symptoms. Thus, we determine whether they required revision surgery.
Sonographic measurement of the thickness of dermis, subcutaneous tissue and pectoralis major muscle: To examine whether the patients present with swelling after an implant-based augmentation mammaplasty, we measure the thickness of dermis, subcutaneous tissue and pectoralis major on breast ultrasound preoperatively and at 1 and 3 months postoperatively (Figure 5).
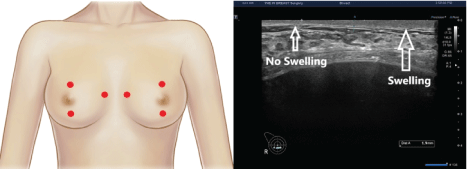
Figure 5: Sonographic measurement of the thickness of dermis, subcutaneous tissue and pectoralis major muscle.
(A) Red dots indicate landmarks for the measurement of the thickness of dermis, subcutaneous tissue and pectoralis major muscle; these include locations 2 cm superior to the upper margin of the areola, 2 cm inferior to the lower margin of the areola and 1 cm medial to the sternal border.
(B) On breast ultrasound, there is an increase in the thickness of dermis in a patient presenting with postoperative swelling.
We evaluated our clinical series of the patients, as previously described. We also analyzed survival of the BellaGel® SmoothFine according to our previous published studies [13,23].
Values were expressed as the number of patients or cases with percentage, Mean ± SD (SD: standard deviation) or Mean ± SE (SE: standard error), where appropriate. The cumulative complication-free survival was estimated, for which 95% confidence intervals (CIs) were provided, followed by the log-rank test. Moreover, the corresponding cumulative complication-free Kaplan-Meier survival and hazards were plotted as a curve. A P-value of <0.05 was considered statistically significant.
Excluding 37 patients, we included a total of 251 women (n=251) in the current study. Their mean age was 31.88 ± 6.83 (20-58) years old. They were followed up during a mean period of 336.51 ± 202.54 (63-911) days. Their baseline characteristics are represented in table 2.
| Variables | Values |
| Age (years old) | 31.88 ± 6.83 (20-58) |
| Sex (male-to-female ratio) | 0:251 |
| Height (cm) | 162.38 ± 5.00 (148-176) |
| Weight (kg) | 50.92 ± 5.39 (40-70) |
| BMI (kg/m2) | 19.29 ± 1.71 (15.62-24.80) |
| FU period (days) | 336.51 ± 202.54 (63-911) |
| Purpose of surgery | |
| Aesthetic | |
| Left breast | 248 (98.8%) |
| Right breast | 249 (99.2%) |
| Reconstructive | |
| Left breast | 2 (0.8%) |
| Right breast | 2 (0.8%) |
| Round of surgery | |
| Primary | |
| Left breast | 250 (99.6%) |
| Right breast | 251 (100.0%) |
| Secondary | 0 (0.0%) |
| Type of incision | |
| Trans-axillary incision | |
| Left breast | 243 (96.8%) |
| Right breast | 243 (96.8%) |
| IMF incision | |
| Left breast | 4 (1.6%) |
| Right breast | 4 (1.6%) |
| Peri-areolar incision | |
| Left breast | 0 (0.0%) |
| Right breast | 0 (0.0%) |
| Others | |
| Left breast | 3 (1.2%) |
| Right breast | 4 (1.6%) |
| Volume of breast implant | |
| <245 cc | |
| Left breast | 4 (1.6%) |
| Right breast | 2 (0.8%) |
| 250-295 cc | |
| Left breast | 62 (24.7%) |
| Right breast | 34 (13.5%) |
| 300-345 cc | |
| Left breast | 143 (57.0%) |
| Right breast | 146 (58.2%) |
| 350-395 cc | |
| Left breast | 35 (13.9%) |
| Right breast | 58 (23.1%) |
| >400 cc | |
| Left breast | 6 (2.4%) |
| Right breast | 10 (4.0%) |
| Profile of breast implant | |
| Ultra-high | |
| Left breast | 0 (0.0%) |
| Right breast | 0 (0.0%) |
| High | |
| Left breast | 234 (93.2%) |
| Right breast | 244 (97.2%) |
| Medium | |
| Left breast | 16 (6.4%) |
| Right breast | 6 (2.4%) |
| Low | |
| Left breast | 0 (0.0%) |
| Right breast | 0 (0.0%) |
| Non-applicable | |
| Left breast | 1 (0.4%) |
| Right breast | 1 (0.4%) |
| Subpectoral pocket | 251 (100.0%) |
| Subglandular pocket | 0 (0.0%) |
| Operation time (min) | 55.12 ± 13.27 |
Table 2: Baseline characteristics of the patients (n=251).
Abbreviations: BMI: body mass index; FU: follow-up; IMF: inframammary fold Values are mean ± standard deviation or the number of cases with percentage, where appropriate.
A total of 48 cases (19.1%) of postoperative complications occurred; these include 22 cases (8.8%) of CC, 8 cases (3.2%) of dissatisfaction with shape, 7 cases (2.8%) of sliding/foreign body sensation, 4 cases (1.6%) of early seroma, 3 cases (1.2%) of early hematoma, 3 cases (1.2%) of infection and 1 case (0.4%) of wound dehiscence (Table 3).
| Variable | Value |
| Early hematoma | 3 (1.2%) |
| Early seroma | 4 (1.6%) |
| CC | 22 (8.8%) |
| Dissatisfaction with shape | 8 (3.2%) |
| Infection | 3 (1.2%) |
| Sliding/foreign body sensation | 7 (2.8%) |
| Wound dehiscence | 1 (0.4%) |
Table 3: Postoperative complications.
Abbreviations: CC, capsular contracture.
Values are the number of the patients with percentage.
In our series, Time-to-Events (TTEs) were estimated at 331.35 ± 12.83 days (95% CI 306.09-356.62) (Table 4). Moreover, cumulative complication-free survival is represented in Table 5. The corresponding Kaplan-Meier cumulative survival and hazards were plotted as a curve (Figures 6 and 7).
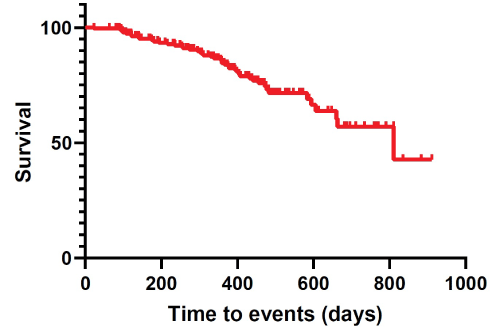
Figure 6: Kaplan-Meier cumulative survival.
In our series, time-to-events were estimated at 331.35 ± 12.83 days (95% CI 306.09-356.62).
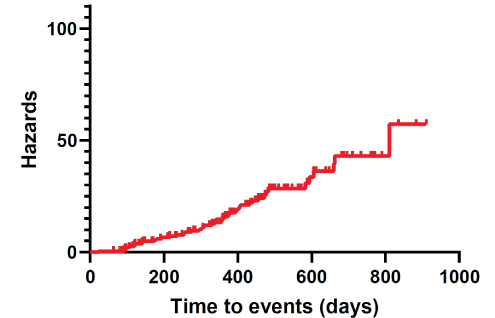
Figure 7: Kaplan-Meier cumulative hazards.
| N | n | Censored value | Time-to-events (months) |
| 251 | 46 | 206 | 331.35 ± 12.83 (306.09-356.62) |
Table 4: Overall complication-free survival.
Note: N: total number of cases; n: incidences of postoperative complications.
Values are mean ± standard error with 95% confidence interval.
| FU | N | n | Survival rate |
| 23 | 251 | 1 | 0.996 ± 0.004 (0.9721-0.9994) |
| 63 | 250 | 0 | 0.996 ± 0.004 (0.9721-0.9994) |
| 80 | 249 | 0 | 0.996 ± 0.004 (0.9721-0.9994) |
| 84 | 248 | 0 | 0.996 ± 0.004 (0.9721-0.9994) |
| 86 | 247 | 0 | 0.996 ± 0.004 (0.9721-0.9994) |
| 87 | 246 | 0 | 0.996 ± 0.004 (0.9721-0.9994) |
| 88 | 244 | 0 | 0.996 ± 0.004 (0.9721-0.9994) |
| 89 | 242 | 0 | 0.996 ± 0.004 (0.9721-0.9994) |
| 90 | 236 | 1 | 0.9918 ± 0.0058 (0.9676-0.9979) |
| 91 | 231 | 0 | 0.9918 ± 0.0058 (0.9676-0.9979) |
| 92 | 223 | 0 | 0.9918 ± 0.0058 (0.9676-0.9979) |
| 93 | 220 | 0 | 0.9918 ± 0.0058 (0.9676-0.9979) |
| 94 | 219 | 0 | 0.9918 ± 0.0058 (0.9676-0.9979) |
| 95 | 218 | 1 | 0.9873 ± 0.0073 (0.961-0.9959) |
| 96 | 216 | 1 | 0.9827 ± 0.0086 (0.9545-0.9935) |
| 97 | 211 | 0 | 0.9827 ± 0.0086 (0.9545-0.9935) |
| 98 | 204 | 0 | 0.9827 ± 0.0086 (0.9545-0.9935) |
| 100 | 202 | 1 | 0.9778 ± 0.0098 (0.9474-0.9907) |
| 101 | 201 | 0 | 0.9778 ± 0.0098 (0.9474-0.9907) |
| 102 | 200 | 0 | 0.9778 ± 0.0098 (0.9474-0.9907) |
| 103 | 199 | 0 | 0.9778 ± 0.0098 (0.9474-0.9907) |
| 105 | 196 | 0 | 0.9778 ± 0.0098 (0.9474-0.9907) |
| 106 | 193 | 0 | 0.9778 ± 0.0098 (0.9474-0.9907) |
| 107 | 191 | 0 | 0.9778 ± 0.0098 (0.9474-0.9907) |
| 108 | 189 | 1 | 0.9778 ± 0.0098 (0.9474-0.9907) |
| 116 | 187 | 0 | 0.9727 ± 0.0111 (0.9399-0.9877) |
| 118 | 186 | 0 | 0.9727 ± 0.0111 (0.9399-0.9877) |
| 119 | 185 | 0 | 0.9727 ± 0.0111 (0.9399-0.9877) |
| 120 | 183 | 0 | 0.9727 ± 0.0111 (0.9399-0.9877) |
| 121 | 182 | 1 | 0.9673 ± 0.0122 (0.9324-0.9843) |
| 122 | 180 | 1 | 0.9619 ± 0.0133 (0.925-0.9809) |
| 132 | 178 | 0 | 0.9619 ± 0.0133 (0.925-0.9809) |
| 133 | 177 | 0 | 0.9619 ± 0.0133 (0.925-0.9809) |
| 140 | 176 | 0 | 0.9619 ± 0.0133 (0.925-0.9809) |
| 141 | 175 | 1 | 0.9564 ± 0.0143 (0.9176-0.9772) |
| 142 | 173 | 1 | 0.9509 ± 0.0152 (0.9103-0.9734) |
| 146 | 172 | 0 | 0.9509 ± 0.0152 (0.9103-0.9734) |
| 148 | 171 | 0 | 0.9509 ± 0.0152 (0.9103-0.9734) |
| 168 | 170 | 0 | 0.9509 ± 0.0152 (0.9103-0.9734) |
| 171 | 169 | 0 | 0.9509 ± 0.0152 (0.9103-0.9734) |
| 174 | 168 | 1 | 0.9452 ± 0.0162 (0.9029-0.9694) |
| 180 | 167 | 1 | 0.9396 ± 0.017 (0.8956-0.9654) |
| 191 | 166 | 0 | 0.9396 ± 0.017 (0.8956-0.9654) |
| 194 | 165 | 1 | 0.9339 ± 0.0179 (0.8884-0.9612) |
| 196 | 164 | 0 | 0.9339 ± 0.0179 (0.8884-0.9612) |
| 210 | 163 | 0 | 0.9339 ± 0.0179 (0.8884-0.9612) |
| 216 | 161 | 1 | 0.9281 ± 0.0187 (0.8811-0.957) |
| 231 | 159 | 0 | 0.9281 ± 0.0187 (0.8811-0.957) |
| 233 | 158 | 0 | 0.9281 ± 0.0187 (0.8811-0.957) |
| 234 | 157 | 0 | 0.9281 ± 0.0187 (0.8811-0.957) |
| 236 | 156 | 1 | 0.9221 ± 0.0195 (0.8737-0.9525) |
| 249 | 155 | 0 | 0.9221 ± 0.0195 (0.8737-0.9525) |
| 254 | 154 | 1 | 0.9162 ± 0.0202 (0.8662-0.948) |
| 257 | 153 | 1 | 0.9102 ± 0.021 (0.8589-0.9434) |
| 266 | 152 | 0 | 0.9102 ± 0.021 (0.8589-0.9434) |
| 270 | 151 | 0 | 0.9102 ± 0.021 (0.8589-0.9434) |
| 274 | 150 | 1 | 0.9041 ± 0.0217 (0.8515-0.9387) |
| 280 | 149 | 0 | 0.9041 ± 0.0217 (0.8515-0.9387) |
| 282 | 148 | 0 | 0.9041 ± 0.0217 (0.8515-0.9387) |
| 284 | 147 | 0 | 0.9041 ± 0.0217 (0.8515-0.9387) |
| 286 | 146 | 0 | 0.9041 ± 0.0217 (0.8515-0.9387) |
| 295 | 145 | 1 | 0.8979 ± 0.0224 (0.8439-0.9339) |
| 302 | 144 | 1 | 0.8916 ± 0.0231 (0.8364-0.929) |
| 306 | 143 | 0 | 0.8916 ± 0.0231 (0.8364-0.929) |
| 307 | 142 | 1 | 0.8854 ± 0.0238 (0.8289-0.924) |
| 311 | 141 | 1 | 0.8791 ± 0.0244 (0.8214-0.919) |
| 323 | 140 | 0 | 0.8791 ± 0.0244 (0.8214-0.919) |
| 330 | 138 | 1 | 0.8727 ± 0.0251 (0.8139-0.9139) |
| 337 | 135 | 0 | 0.8727 ± 0.0251 (0.8139-0.9139) |
| 338 | 134 | 1 | 0.8662 ± 0.0257 (0.8062-0.9087) |
| 343 | 133 | 0 | 0.8662 ± 0.0257 (0.8062-0.9087) |
| 350 | 132 | 0 | 0.8662 ± 0.0257 (0.8062-0.9087) |
| 355 | 130 | 1 | 0.8595 ± 0.0264 (0.7983-0.9033) |
| 357 | 129 | 0 | 0.8595 ± 0.0264 (0.7983-0.9033) |
| 358 | 128 | 1 | 0.8528 ± 0.027 (0.7905-0.8978) |
| 359 | 127 | 1 | 0.8461 ± 0.0276 (0.7826-0.8923) |
| 363 | 125 | 0 | 0.8461 ± 0.0276 (0.7826-0.8923) |
| 364 | 124 | 0 | 0.8461 ± 0.0276 (0.7826-0.8923) |
| 365 | 122 | 0 | 0.8461 ± 0.0276 (0.7826-0.8923) |
| 366 | 118 | 1 | 0.8389 ± 0.0283 (0.7742-0.8865) |
| 367 | 114 | 0 | 0.8389 ± 0.0283 (0.7742-0.8865) |
| 369 | 112 | 0 | 0.8389 ± 0.0283 (0.7742-0.8865) |
| 371 | 111 | 0 | 0.8389 ± 0.0283 (0.7742-0.8865) |
| 372 | 108 | 0 | 0.8389 ± 0.0283 (0.7742-0.8865) |
| 374 | 107 | 0 | 0.8389 ± 0.0283 (0.7742-0.8865) |
| 375 | 106 | 1 | 0.831 ± 0.0291 (0.7647-0.8801) |
| 378 | 105 | 1 | 0.8231 ± 0.0299 (0.7553-0.8737) |
| 379 | 103 | 0 | 0.8231 ± 0.0299 (0.7553-0.8737) |
| 385 | 102 | 0 | 0.8231 ± 0.0299 (0.7553-0.8737) |
| 386 | 101 | 0 | 0.8231 ± 0.0299 (0.7553-0.8737) |
| 388 | 100 | 0 | 0.8231 ± 0.0299 (0.7553-0.8737) |
| 389 | 99 | 0 | 0.8231 ± 0.0299 (0.7553-0.8737) |
| 391 | 97 | 0 | 0.8231 ± 0.0299 (0.7553-0.8737) |
| 392 | 96 | 0 | 0.8231 ± 0.0299 (0.7553-0.8737) |
| 394 | 95 | 1 | 0.8144 ± 0.0308 (0.7448-0.8667) |
| 399 | 94 | 1 | 0.8058 ± 0.0317 (0.7345-0.8597) |
| 404 | 93 | 1 | 0.7971 ± 0.0325 (0.7243-0.8526) |
| 407 | 92 | 1 | 0.7884 ± 0.0333 (0.7141-0.8455) |
| 408 | 91 | 0 | 0.7884 ± 0.0333 (0.7141-0.8455) |
| 422 | 89 | 0 | 0.7884 ± 0.0333 (0.7141-0.8455) |
| 424 | 88 | 0 | 0.7884 ± 0.0333 (0.7141-0.8455) |
| 426 | 87 | 0 | 0.7884 ± 0.0333 (0.7141-0.8455) |
| 430 | 85 | 0 | 0.7884 ± 0.0333 (0.7141-0.8455) |
| 432 | 84 | 1 | 0.7791 ± 0.0342 (0.703-0.8379) |
| 433 | 83 | 0 | 0.7791 ± 0.0342 (0.703-0.8379) |
| 435 | 81 | 0 | 0.7791 ± 0.0342 (0.703-0.8379) |
| 438 | 80 | 0 | 0.7791 ± 0.0342 (0.703-0.8379) |
| 441 | 79 | 1 | 0.7692 ± 0.0351 (0.6913-0.8298) |
| 442 | 78 | 0 | 0.7692 ± 0.0351 (0.6913-0.8298) |
| 447 | 77 | 0 | 0.7692 ± 0.0351 (0.6913-0.8298) |
| 448 | 76 | 0 | 0.7692 ± 0.0351 (0.6913-0.8298) |
| 449 | 75 | 0 | 0.7692 ± 0.0351 (0.6913-0.8298) |
| 450 | 74 | 0 | 0.7692 ± 0.0351 (0.6913-0.8298) |
| 454 | 73 | 0 | 0.7692 ± 0.0351 (0.6913-0.8298) |
| 456 | 72 | 1 | 0.7585 ± 0.0362 (0.6785-0.8212) |
| 457 | 69 | 0 | 0.7585 ± 0.0362 (0.6785-0.8212) |
| 458 | 68 | 0 | 0.7585 ± 0.0362 (0.6785-0.8212) |
| 459 | 67 | 0 | 0.7585 ± 0.0362 (0.6785-0.8212) |
| 461 | 66 | 0 | 0.7585 ± 0.0362 (0.6785-0.8212) |
| 464 | 65 | 0 | 0.7585 ± 0.0362 (0.6785-0.8212) |
| 466 | 62 | 0 | 0.7585 ± 0.0362 (0.6785-0.8212) |
| 467 | 61 | 0 | 0.7585 ± 0.0362 (0.6785-0.8212) |
| 468 | 59 | 0 | 0.7585 ± 0.0362 (0.6785-0.8212) |
| 469 | 58 | 0 | 0.7585 ± 0.0362 (0.6785-0.8212) |
| 470 | 57 | 0 | 0.7585 ± 0.0362 (0.6785-0.8212) |
| 471 | 56 | 0 | 0.7585 ± 0.0362 (0.6785-0.8212) |
| 472 | 55 | 1 | 0.7447 ± 0.0381 (0.6608-0.8108) |
| 474 | 54 | 0 | 0.7447 ± 0.0381 (0.6608-0.8108) |
| 475 | 53 | 0 | 0.7447 ± 0.0381 (0.6608-0.8108) |
| 477 | 52 | 1 | 0.7304 ± 0.04 (0.6427-0.7999) |
| 482 | 51 | 0 | 0.7304 ± 0.04 (0.6427-0.7999) |
| 483 | 50 | 1 | 0.7304 ± 0.04 (0.6427-0.7999) |
| 486 | 49 | 0 | 0.7158 ± 0.0418 (0.6245-0.7886) |
| 487 | 48 | 0 | 0.7158 ± 0.0418 (0.6245-0.7886) |
| 492 | 47 | 0 | 0.7158 ± 0.0418 (0.6245-0.7886) |
| 494 | 46 | 0 | 0.7158 ± 0.0418 (0.6245-0.7886 |
| 497 | 45 | 0 | 0.7158 ± 0.0418 (0.6245-0.7886) |
| 500 | 43 | 0 | 0.7158 ± 0.0418 (0.6245-0.7886) |
| 502 | 42 | 0 | 0.7158 ± 0.0418 (0.6245-0.7886) |
| 503 | 41 | 0 | 0.7158 ± 0.0418 (0.6245-0.7886) |
| 510 | 39 | 0 | 0.7158 ± 0.0418 (0.6245-0.7886) |
| 512 | 37 | 0 | 0.7158 ± 0.0418 (0.6245-0.7886) |
| 526 | 36 | 0 | 0.7158 ± 0.0418 (0.6245-0.7886) |
| 536 | 34 | 0 | 0.7158 ± 0.0418 (0.6245-0.7886) |
| 547 | 33 | 0 | 0.7158 ± 0.0418 (0.6245-0.7886) |
| 563 | 32 | 0 | 0.7158 ± 0.0418 (0.6245-0.7886) |
| 566 | 31 | 0 | 0.7158 ± 0.0418 (0.6245-0.7886) |
| 568 | 30 | 0 | 0.7158 ± 0.0418 (0.6245-0.7886) |
| 572 | 29 | 0 | 0.7158 ± 0.0418 (0.6245-0.7886) |
| 583 | 28 | 1 | 0.6902 ± 0.0475 (0.5867-0.7728) |
| 588 | 27 | 0 | 0.6902 ± 0.0475 (0.5867-0.7728) |
| 594 | 26 | 1 | 0.6637 ± 0.0525 (0.5496-0.7552) |
| 606 | 25 | 1 | 0.6371 ± 0.0567 (0.5148-0.7364) |
| 612 | 23 | 0 | 0.6371 ± 0.0567 (0.5148-0.7364) |
| 613 | 22 | 0 | 0.6371 ± 0.0567 (0.5148-0.7364) |
| 638 | 21 | 0 | 0.6371 ± 0.0567 (0.5148-0.7364) |
| 648 | 20 | 0 | 0.6371 ± 0.0567 (0.5148-0.7364) |
| 660 | 19 | 1 | 0.6036 ± 0.0629 (0.4693-0.714) |
| 663 | 18 | 1 | 0.5701 ± 0.0678 (0.4271-0.6898) |
| 681 | 17 | 0 | 0.5701 ± 0.0678 (0.4271-0.6898) |
| 682 | 16 | 0 | 0.5701 ± 0.0678 (0.4271-0.6898) |
| 687 | 15 | 0 | 0.5701 ± 0.0678 (0.4271-0.6898) |
| 695 | 14 | 0 | 0.5701 ± 0.0678 (0.4271-0.6898) |
| 711 | 13 | 0 | 0.5701 ± 0.0678 (0.4271-0.6898) |
| 734 | 12 | 0 | 0.5701 ± 0.0678 (0.4271-0.6898) |
| 760 | 11 | 0 | 0.5701 ± 0.0678 (0.4271-0.6898) |
| 765 | 10 | 0 | 0.5701 ± 0.0678 (0.4271-0.6898) |
| 771 | 8 | 0 | 0.5701 ± 0.0678 (0.4271-0.6898) |
| 791 | 6 | 0 | 0.5701 ± 0.0678 (0.4271-0.6898) |
| 810 | 5 | 0 | 0.5701 ± 0.0678 (0.4271-0.6898) |
| 811 | 4 | 1 | 0.4275 ± 0.1335 (0.1745-0.6613) |
| 835 | 3 | 0 | 0.4275 ± 0.1335 (0.1745-0.6613) |
| 883 | 2 | 0 | 0.4275 ± 0.1335 (0.1745-0.6613) |
| 911 | 1 | 0 | 0.4275 ± 0.1335 (0.1745-0.6613) |
Table 5: Cumulative complication-free survival.
Note: FU: follow-up; N: total number of cases; n: incidences of postoperative complications
Values are mean ± standard error with 95% confidence interval.
Representative cases are presented in Figures 8 and 9.
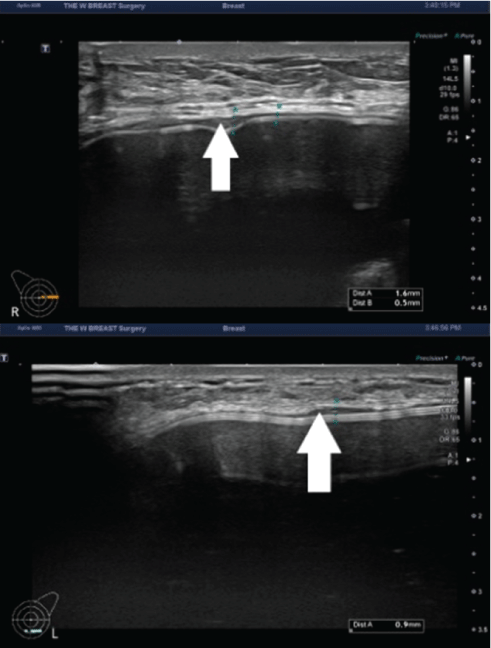
Figure 8: The capsule thickness measured at 3 months postoperatively.
A 48-year-old woman received augmentation mammaplasty using the BellaGel® SmoothFine via a trans-axillary incision in the subpectoral pocket. At 3 months postoperatively, the patient presented with thickened capsule (white arrow) in both breasts; the capsule thickness was measured as 0.3-1.6 mm in the right breast (A) and 0.3-0.9 mm in the left breast (B).
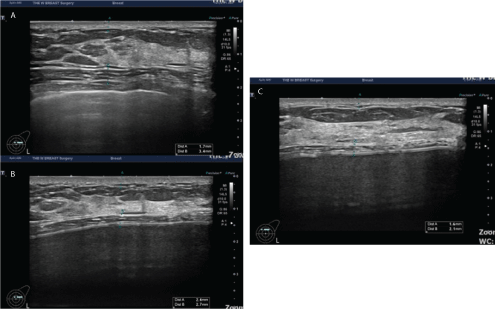
Figure 9: The thickness of the dermis measured preoperatively and at 1 and 3 months postoperatively.
A 28-year-old woman received augmentation mammaplasty using the BellaGel® SmoothFine via a trans-axillary incision in the subpectoral pocket. In this patient, the thickness of the dermis was measured preoperatively and at 1 and 3 months postoperatively. It was measured as 1.7 mm preoperatively (A), 2.4 mm at 1 month (B) and 1.6 mm at 3 months (C).
In an effort to obtain predictable and stable outcomes of an implantbased augmentation mammaplasty, a detailed preoperative assessment is a mandatory procedure. This requires selection of appropriate patients and devices [24]. In more detail, optimal breast implants should be selected considering both their shape and volume and variations in the morphological characteristics of the breast, which is within the scope of well-established, evidence-based protocols [12,25,26]. From this context, preoperative planning for an implantbased augmentation mammaplasty provides useful information about volume and shape of a device through an individualized application of a 3-D imaging modality [27]. Thus, a 3-D imaging modality is more advantageous in quantifying differences in anthropometric variables, such as the shape, dimension and volume of the breast, between preoperatively and postoperatively as compared with a 2-D photography or physical examination [12,28]. This enables a surgeon to simulate possible postoperative appearance of the implant placed in a patient’s body. The resulting improved communication between the two parties would raise the degree of patient satisfaction with outcomes of an implant-based augmentation mammaplasty [27].
Capsules around the breast implant are characterized by the presence of specialized fibroblasts which are capable of contracting themselves, thus termed as contractile fibroblasts, and then presumed to be responsible for CC [29,30]. These contractile fibroblasts, or myofibroblasts, were first described in wound granulation tissue and their involvement in wound contraction has also been described [31,32]. It is also known that they disappear with wound healing, but their persistent presence in abnormal scars, such as hypertrophic scar and keloid. They are also found in such conditions as fibrosis, such as Dupuytren’s contracture and desmoid tumor [33,34]. Moreover, they play a role in forming the fibrous stroma in response to epithelial and lymphatic malignancy [35-37].
The myofibroblasts in an implant capsule are histopathologically characterized by parallel arrangement of fibrillar bundles of 4-8 nm in diameter to their axis, nuclear deformations composed of folding of the nuclear membrane, surface differentiation featuring the parallel arrangement of an extracellular layer of fibrils to them and cellular interconnections. This is different from normal fibroblasts and shares some characteristics with smooth muscle cells. Thus, such myofibroblasts are therefore coined based on their structural and functional features; they are equipped with a capability of contracting themselves and applying the force to wound contraction [38,39].
In a large number of literatures, effects on fibroblast differentiation are commonly measured at 3 months [40-44]. This can be seen in the context of effects of leukotriene antagonists on CC. Zafirlukast (Accolate®; AstraZeneca Pharmaceuticals, Wilmington, DE) and montelukast (Singulair®; Merck Sharp & Dohme, Whitehouse Station, NJ) are leukotriene antagonists that are effective in reversing CC following augmentation mammaplasty because of their inhibitory actions on cysteinyl leukotrienes and myofibroblasts, both of which cause CC [45,46].
Application of breast ultrasound to an early detection of its complications is a useful modality. Moreover, a follow-up time point of 3 months postoperatively is minimally necessary to detect the CC. This is plausible from evidence-based perspectives; Reid RR, et al. [47] reported that there were favorable responses to the treatment of early CC with zafirlukast in patients who received an implant-based primary submuscular augmentation mammaplasty. According to these authors, 75.7% of the patients receiving treatment had a complete or partial response to zafirlukast at 3 months. Therefore, we routinely perform breast ultrasound to examine whether patients receiving a breast implant present with any notable signs and symptoms of complications at 3 months postoperatively
Women receiving an implant-based augmentation mammaplasty may be at a risk of developing diverse types of early (e.g., formation of seroma and hematoma) and late complications (e.g., CC, gel bleed and rupture). Early complications may eventually lead to extrusion of a device. Late complications are considered an implant failure [48,49]. Of these, seroma formation is definitely recognized as a complication in women whose capsule is left intact. In addition, late seroma is defined as a notable accumulation of serous fluid, such as exudate or effusion, in the capsule seen at least 12 months of an implant-based augmentation mammaplasty [50,51]. It is such a rare entity that its incidence is estimated at approximately 0.88-1.84% [52,53]. Moreover, its relationship with textured implants has been well described in the literature [54]. Its main clinical presentations include a sudden progressive swelling of the breast and discomfort [49].
In the current study, there were a total of 48 cases (19.1%) of postoperative complications. Of these, CC occurred at an incidence of 8.8%. This is noteworthy because manufacturer-sponsored retrospective studies have shown incidences of 0.0% (0/239), 0.6% (1/78) and 1.6% (10/621) in patients receiving the BellaGel® implants including the BellaGel® SmoothFine [14,19,20]. Interestingly, another manufacturer-sponsored experimental study compared the vulnerability to CC based on surface properties between the BellaGel® implants, including the BellaGel® SmoothFine, and the Motiva Ergonomix™ SilkSurface (Establishment Labs Holdings Inc., Alajuela, Costa Rica), thus drawing conclusions that the BellaGel® SmoothFine was the least vulnerable to CC of the sample devices [18]. According to a recent non-manufacturersponsored study comparing the 1-year safety between the BellaGel® SmoothFine and the Motiva Ergonomix™, however, CC occurred at incidences of 2.27% (6/264) and 0.00% (0/76), respectively [23]. This strongly indicates not only that result of manufacturer-sponsored studies should be interpreted with caution but also that use of high-resolution ultrasound is an essential tool for accurately detecting complications of an implant-based augmentation mammaplasty at the earliest opportunities possible.
Postoperative swelling after aesthetic and reconstructive implantbased augmentation mammaplasty is not an uncommon entity. Its common causes include hematoma, seroma and infection [52,54-58]. Accumulation of fluid around the breast implant may serve as a cause of complications such as infection, implant extrusion, tissue necrosis, poor wound healing, inhibition of tissue growth into scaffolds and distortion of the size and shape of the implant. It would therefore be mandatory to make a prompt, accurate diagnosis of postoperative swelling; fluid should be collected from the adjacent areas to the breast implant and then cultured, which should be followed by treatment with antibiotics. Moreover, use of specialized diagnostic as well as surgical modalities with needle-guided imaging is currently recommended for the treatment of relevant cases [59]. But there were no cases of postoperative swelling in our series.
A recent manufacturer-sponsored study compared the safety between the breast implants from 6 different manufacturers in Korea; Yoon S and Chang JH compared 1-year safety outcomes between the BellaGel®/BellaGel® SmoothFine (n=182), the Mentor CPG™ (Mentor Worldwide LLC, Santa Barbara, CA) (n=159), the Motiva Ergonomix™ (n=152), the Matrix™ (GC Aesthetics PLC, Apt Cedex, France) (n=135), the Naturgel® (Groupe Sebbin SAS, Boissy-l’Aillerie, France) (n=61) and the Natrelle® 410/510 (Allergan Inc., Irvine, CA) (n=20). These authors reported that the safety of the BellaGel® SmoothFine is not inferior to its competitors [19]. But this warrants more objective, evidence-based studies. Kim JH previously reported not only that the HansBiomed Co. Ltd., the manufacturer of the BellaGel® SmoothFine, illegally used unapproved substances, such as 7-9700 and Q7-4850, for manufacturing of it but also that the manufacturer deliberately modified its shell structure in violation of the regulatory requirement enforced by the KMFDS [21,22]. It is therefore impossible to completely rule out the possibility that patients receiving the BellaGel® SmoothFine containing hazardous substances or the modified shell structure.
We, at the Korean Society of Breast Implant Research, propose the following recommendations: First, a patient registry should be considered as an infrastructure for the standardized recording of data from patients receiving the BellaGel® implants. In 2019, when we noticed the first report of the Korean case of breast implant-associated anaplastic large cell lymphoma (BIA-ALCL), we proposed that possible impacts of BIA-ALCL be rigorously analyzed and appropriate measures be taken as promptly as possible [60]. From similar contexts, we’ll prospectively collect from patients receiving the BellaGel® implants and then track their outcomes and complications, thus endeavoring to ensure both high-quality care and patient safety. This should be accompanied by collaborations between patients receiving the BellaGel® implants and the KMFDS. Second, patients receiving the BellaGel® implants should be meticulously monitored for rupture of the device. Lack of early detection of rupture of a breast implant may cause patients to be vulnerable to silicone-induced axillary lymphadenopathy as well as extracpasular rupture [61,62]. This is serious because a complete resection of the mammary tissue involving silicone would not be achieved, a diagnosis of rupture of the device may be missed due to the residual presence of silicone even after explantation and silicone compounds may be present in breast milk [63,64]. Third, patients receiving the BellaGel® SmoothFine should be meticulously evaluated for possible detrimental effects due to an unknown number of those with the 4-layered device.
To summarize, our results are as follows:
1) A total of 48 cases (19.1%) of postoperative complications occurred; these include 22 cases (8.8%) of CC, 8 cases (3.2%) of dissatisfaction with shape, 7 cases (2.8%) of sliding/foreign body sensation, 4 cases (1.6%) of early seroma, 3 cases (1.2%) of early hematoma, 3 cases (1.2%) of infection and 1 case (0.4%) of wound dehiscence.
2) TTEs were estimated at 331.35 ± 12.83 days (95% CI 306.09- 356.62).
But limitations of the current study are as follows: First, we failed to perform a sufficiently long-term follow-up of our cohorts to verify whether a time point of 3 months postoperatively would be minimally necessary to detect the CC. Further large-scale, long-term follow-up studies and more evidence-based efforts are warranted to validate our approaches. Second, we failed to evaluate our clinical series of the patients under the prospective design. Prospective studies, including randomized controlled trials in particular, are more reliable in showing more scientifically sound approaches and valuable outcomes as compared with retrospective ones. It is difficult, however, to conduct prospective studies [65]. Third, there were 22 cases (8.8%) of CC in our series, which cannot be generalized because of a short length of follow-up. According to a 10-year prospective study conducted by Maxwell GP, et al. [66,67] there was an approximately 1% increase in the incidence of CC of Baker grade III/IV from that described in their previous 6-year “Core” study. It can therefore be inferred that the incidence of CC might further rise over time. Fourth, we failed to quantify aesthetic outcomes using preoperative anthropometric measurements obtained on the Divina™ 3-D Scanner. Fifth, we failed to assess the value of the thickness of dermis, subcutaneous tissue and pectoralis major as indicators of postoperative swelling.
Here, we describe 3-year safety outcomes of an implant-based augmentation mammaplasty using the BellaGel® SmoothFine in Korean women. But the KFDS and surgeons should perform a meticulous long-term follow-up of patients receiving the BellaGel® SmoothFine and then consider the possibility that they might be vulnerable to its possible detrimental effects. According to a recent study, the BellaGel® SmoothFine and the Motiva Ergonomix™ are popular brands of a microtextured breast implant in Korea. From our empirical experience, however, the BellaGel® SmoothFine is not preferable to the Motiva Ergonomix™; the former shows a higher rate of CC, as shown in the current study, and its profile is lower as compared with the latter [23]. In this regard, the Motiva Ergonomix™ is a device of choice for Korean women receiving the BellaGel® SmoothFine.
This is a non-manufacturer-sponsored study.
This study was supported by the Korean Society of Breast Implant Research.
The authors have nothing to declare in relation to the current work.
All procedures performed in this study were in accordance with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Written informed consent was waived due to the retrospective nature of the current study.
Download Provisional PDF Here
Article Type: RESEARCH ARTICLE
Citation: Song KY, Sung JY, Choi WS, Lim HG, Kim JH (2021) An Ultrasound-Assisted Approach to an Early Detection of Complications of an Implant-Based Augmentation Mammaplasty using the BellaGel® SmoothFine: Preliminary 3-year Clinical Experience. J Surg Open Access 7(2): dx.doi.org/10.16966/2470-0991.232
Copyright: © 2021 Song KY, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Publication history:
All Sci Forschen Journals are Open Access