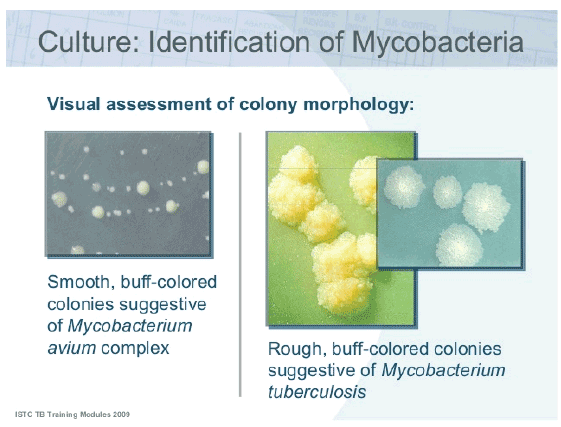
Figure 1: Images showing the differences in colony morphology between Mycobacterium Avium and Mycobacterium tuberculosis.

Neelam Patel1* Ranganathan B2 Faris C3 Kumar N 2
1Foundation Year 2 Doctor, Wrightington, Wigan and Leigh Trust, Wigan Lane, Wigan, Lancashire, UK*Corresponding author: Neelam Patel, Foundation Year 2 Doctor, Wrightington, Wigan and Leigh Trust, Wigan Lane, Wigan, Lancashire, UK, Tel: 07859904753; E-mail: Neelam.patel@wwl.nhs.uk
Neck lumps are a very common presentation to hospital in pediatric patients. Lymphadenitis secondary to atypical mycobacterium infections are rare but recently there has been an increase in prevalence in the UK. The treatment of such cases is controversial; the options of medical, surgical or combination of both are practiced, with variations, in different centres around the world. We report a case of a 4 year old girl who presented with a short history of a neck lump due to Mycobacterium Avium Complex (MAC) infection which was surgically excised, with good post-op and aesthetic outcome. This article aims to educate upon the background, clinical features and management of patients presenting with MAC associated neck lumps.
Mycobacterium avium complex; Nontuberculous mycobacteria; Cervical lymphadenopathy; Lymphadenitis
Nontuberculous mycobacterial (NTM) infections are relatively rare, however the incidence of these infections in the UK has been steadily increasing over the past couple of decades, emphasising the need for greater awareness of these organisms as pathogens [1,2]. MAC commonly affects those that are immune compromised, especially in AIDS, where M. Avium has been shown to cause disseminated disease. However, the rate of mycobacterial infections in immune competent individuals seems to be increasing independently. MAC causes pulmonary disease, especially in older persons with or without underlying lung disease and patients with cystic fibrosis. In patients with pre-existing lung disease, the common extra pulmonary site of presentation of those with MAC infections manifest as cervical lymphadenitis.
MAC cervical lymphadenitis is common in age groups between 1-5 years usually affecting parotid and submandibular group of lymphnodes. A clinical diagnosis is confirmed by culture or polymerase chain reaction; organisms can be isolated by culture in 50% of cases [3].
We illustrate the case of a 4 year old child presenting to clinic with a right sided neck swelling noticed after an acute episode of sore throat shortly after Christmas. On examination, there was a non-tender neck lump, measuring 3 cm at right submandibular triangle; it was firm and mobile, had no fluctuance, no associated erythema and no other associated lymphadenopathy. An initial ultrasound was performed showing a lump with areas of reduced echogenicity and cystic spaces. This mass was suggested to represent an enlarged breaking down node and measured 3.5 cm × 2.5 cm. The patient was otherwise fit and well with no acute symptoms. As clinically the lump did not appear to be an abscess, she was continued on antibiotics with a plan to review in outpatient clinic.
A few weeks later, the patient was reviewed in the clinic, swelling had subsided slightly after the course of antibiotics; however as the neck swelling was persistent a further ultrasound scan was arranged which showed no significant change in size but the appearance was not typical of reactive lymphadenitis. In view of persistence of neck lump and ultrasound finding an excision and biopsy was performed.
Patient was reviewed post operatively in the outpatient clinic. The histopathology report showed that the lymphnode’s architecture was almost entirely effaced by well-defined granulomas composed of epithelioid macrophages, including Langerhans-type giant cells, with many of the granulomas containing a central area of necrosis. The morphological appearance was in fact highly suggestive of tuberculosis, even though microscopy using the Ziehl-Neelson stain failed to show any acid fast bacilli. The tissue culture however did show a growth of Mycobacterium avium complex which was sensitive to various antimicrobials.
The patient was referred to the respiratory consultant who is a lead for tuberculosis in our hospital trust. Since the child was not immunocompromised and clinically did not have any systemic signs and symptoms, with no signs of recurrence in the neck, it was decided to manage it conservatively by monitoring regularly in the outpatient clinic.
She was reviewed again by the ENT consultants post-op where she was making a good recovery following the operation, the wound was clean and the scar aesthetically pleasing and there were no signs of recurrence of the disease after eight months of follow up in the clinic.
Nontuberculous mycobacteria (NTM) are a group of acid-fast bacteria that can be isolated from water, soil, food products, bathrooms (shower heads), cigarette components and domestic and wild animals. Nosocomial transmission has also occurred with medical equipment [4].
There have been over 130 opportunistic pathogens that can infect humans including, Mycobacterium avium complex (MAC), M. marinum and M kansasii. Mycobacterium avium and Mycobacterium intracellulare are difficult to differentiate between the two and so are grouped as MAC [5,6].
In terms of prevalence, studies have shown there to be an obvious agerelated difference in aetiology of mycobacterial cervical lymphadenopathy. A study carried out by Lai et al. [7] showed that out of the 147 instances of mycobacterial cervical lymphadenopathy identified in adults, only seven nodes contained atypical mycobacteria, with the others containing M tuberculosis. Compare this to mycobacterial cervical lymphadenopathy in children, where 55 of the 60 patients identified had an atypical mycobacteria present in the nodes and five M. tuberculosis identified in the others, further emphasising the prevalence of atypical mycobacteria in children.
The true incidence or prevalence of MAC lymphadenitis in the UK is unknown as this is not a notifiable disease.
The mode of infection is thought to be through oral contact with MAC infected water [8]. The frequent involvement of cervical lymph nodes suggests that ingestion and direct tissue penetration by the organism is the usual pathogenesis of NTM lymphadenitis [9].
One of the classical presenting features of a MAC infection is a firm, painless, unilateral cervico facial mass and the patient usually has a trial of multiple antibiotics with no significant improvement. This mass can then become fluctuant and enlarge. Commonly the lump is evident in the submandibular region, but also in the pre-auricular and parotid areas and usually with an absence of fever and are systemically well. Following unsuccessful antimicrobial therapy, these lesions typically progress into liquefaction with violaceous discoloration of the overlying skin and then spontaneous drainage through the skin [3].
More recently, extra pulmonary manifestations of NTM have been linked with a defect in the IFN-γ and interleukin-12 signaling. Previous documented case reports have shown the presence of auto antibodies against IFN-γ and having a lower interferon gamma production therefore being a potential cause of the patient’s rare, acquired immunodeficiency [10,11]. In addition, therapy with anti-TNF alpha treatment has been shown to be a risk factor for NTM disease, again with the most common causative bacteria being the Mycobacteria avium [12].
There are multiple differentials of a unilateral neck lump, including: other infectious causes (tuberculous disease, toxoplasmosis, viral (cytomegalovirus, Ebstein Barr virus), Staphylococcus aureus and Streptococcus pyogenes, and Bartonella henselae- the cat scratch disease agent); and non-infectious causes (lymphoma, Kawasaki disease, branchial cysts and congenital neck masses).
In terms of imaging, ultrasound is generally the best method for investigating these kinds of neck lumps as minimising the radiation exposure is in the best interests of pediatric patients. However, CT scans would allow a more detailed view and give a better impression of the surrounding anatomy, according to a study by Robson et al. [13] the NTM infection would show a characteristic appearance. Their key findings were that the infection would show as an asymmetric adenopathy with contiguous low-density ring enhancing masses, which was present in all the patients analysed.
Diagnosis of NTM adenitis is determined by the clinical presentation, tuberculin skin testing, mycobacterial cultures and, to a lesser extent, histology and imaging.
Clinical presentation of children with NTM lymphadenitis has been discussed above.
Tuberculin skin testing may help diagnosis but depends upon previous exposure to M tuberculosis infections and receipt of BCG vaccine, testing with M tuberculosis purified protein derivative resulting in an in duration greater than 5 mm at 48 hours supports a diagnosis of NTM infection [14]. Unfortunately, the use of NTM skin test reagents is not currently available for clinical use [15].
Definitive diagnosis of NTM lymphadenitis requires isolation of NTM from culture of tissues, aspirated fluid or pus; swabs are specimens of a lesser quality. Specimens for microbiologic and histopathologic investigations may be obtained through excisional biopsy, fine needle aspiration or from fistulous drainage. Incision and drainage generally is contraindicated if NTM is suspected because of the risk of recurrence or development of a chronically draining fistula. Microscopy by fluorochrome method (auramine stain) is preferred for microscopic recognition of NTM in clinical samples. However the appearance of NTM by microscopy is often indistinguishable from that of M tuberculosis and negative acid fast bacilli stains do not exclude mycobacterial aetiology. Conventional culture based techniques can take as long as eight weeks to isolate a mycobacterial species from a clinical specimen but currently no other test is as sensitive [15].
As a consequence of the demand for more rapid diagnosis of M tuberculosis, identification of NTM increasingly focuses on the use of genotypic methods for the rapid identification of mycobacterial species from cultured isolates (gene probes or PCR) but also on the detection of mycobacteria directly from clinical specimens, by alternative nucleic acid amplification tests (Figure 1).

Figure 1: Images showing the differences in colony morphology between Mycobacterium Avium and Mycobacterium tuberculosis.
Studies have shown that untreated MAC in HIV significantly shortens survival [16]. Various multi-agent regimes are available for treatment but there is no clear evidence showing benefits of the role of medical therapy alone in the treatment of MAC associated neck lumps and actually have an inferior outcome [17].
In contrast, there are multiple articles on the role of surgery for the treatment of MAC associated neck lumps. A study performed by Schaad et al. [18] reviewed 380 patients treated for atypical mycobacterial adenitis and showed surgical excision to be the treatment of choice. The results showed a 92% cure rate with total surgical excision alone and furthermore a more improved 95% where excision was followed by antituberculous drug therapy. In contrast, only 16% were cured where an incision drainage was performed and only one out of the ten patients treated with antituberculous drugs alone was cured. Therefore showing total surgical excision to be the treatment of choice in these group of patients [18]. Moreover, this has been supported by various studies looking into optimal management plan of pediatric cases of atypical mycobacterial lymphadenitis, showing that the best option is a surgical excision biopsy ± postoperative antibiotics to be the optimal treatment choice [5,19,20].
Surgery can be challenging, especially where there is extensive involvement of the skin and also risk of permanent facial nerve injury which can give permanent lifelong disfigurement. The facial nerve can lie very superficial over the parotid region and so is at increased risk during surgery, to prevent potential nerve damage an intraoperative nerve monitor can be utilised. Furthermore, if surgery is considered, every effort should be made to place the incision on a healthy uninvolved skin region as this will give rise to a superior cosmetic outcome and also helps with the process of wound healing. Notably, referral to specialist tertiary centre should be considered in patients with extensive skin involvement for a better outcome.
Download Provisional PDF Here
Article Type: Case Report
Citation: Patel N, Ranganathan B, Faris C, Kumar N (2016) Case Presentation - Atypical Mycobacterial Cervical Lymphadenitis. J Surg 2(4): doi http:// dx.doi.org/10.16966/2470-0991.118
Copyright: © 2016 Patel N, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Publication history:
All Sci Forschen Journals are Open Access