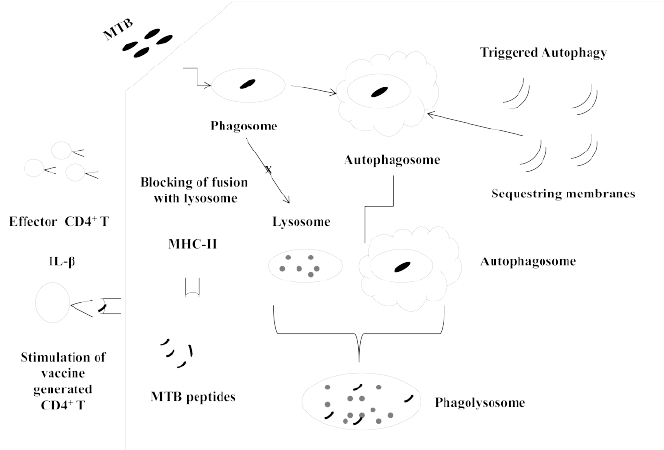
Figure 1: Cartoon depicting various steps involving towards stimulation of memory generated CD4+ Th1 cells through induction of autophagy in antigen presenting cells (APCs)


Om Parkash*
Immunology Laboratory, National JALMA Institute for Leprosy and Other Mycobacterial Diseases, Tajganj, Agra, India*Corresponding author: Om Parkash, Immunology Laboratory, National JALMA Institute for Leprosy and Other Mycobacterial Diseases, Tajganj, Agra 282004, India, Tel: +91 562 2231248; E-mail: om1234@gmail.com; op_v3@yahoo.com
Despite extensive efforts made towards developing anti tuberculosis (TB) vaccine, no effective vaccine better than BCG has emerged as yet. This could be due to failure of the professional antigen presenting cells (APCs; monocytes/macrophages and dendritic cells) in killing the internalized infecting Mycobacterium tuberculosis (MTB) and thereafter in processing and presentation (in association with MHC-II) of MTB derived antigens to stimulate the vaccine generated memory CD4 Th1 cells against MTB. It is well documented in the literature that autophagy can be triggered in APCs to kill invading MTB where-after MTB derived antigens could be processed and presented to stimulate anti TB vaccine generated memory CD4 Th1 cells to induce cell mediated immunity for protection of the host. In this communication, a view has been expressed wherein repetitive induction (at some time intervals) of autophagy in APCs may help in sustaining the anti TB vaccine generated protective immunity against infecting MTB bacilli.
Tuberculosis; Vaccine; Autophagy; Leprosy; AIDS
T-bet: T-box Transcription Factor; RORγt: Retinoid-acid R eceptor-related Orphan Receptor gamma t
Tuberculosis (TB) is a contagious airborne disabling disease caused, primarily, by an intracellular infectious pathogen, Mycobacterium tuberculosis (MTB). It is considered to be a leading, next to Acquired Immuno Deficiency Syndrome (AIDS), cause of deaths due to any single infectious agent. The data from the latest report of World Health Organization [1] reflect that TB is the deadliest cause of public health concern. Since there is no available efficient anti TB vaccine, intensive efforts are being made, employing anti TB drugs, to overcome TB as a global health problem. However, emergence of drug-resistant (multi drug resistant and extensively drug resistant and even total drug resistant, for which effective drugs are virtually not available) MTB strains and TB-HIV co-infection appears to be big hindrances in achieving target for global control of TB [1] by treatment with available drugs. The costs involved towards: (i) diagnosis of TB (ii) detection of drug resistant TB cases (iii) acquiring of anti TB drugs (for both, drug sensitive and drug resistant TB) and (iv) delivery of drugs to TB patients; have also become big economic threats making TB control prohibitive, especially for the developing countries. The expenses towards managing the global TB epidemic by TB treatment itself is estimated to be more than 8 billion US dollars per year. Further, large majority of TB cases belong to the working age (15-54 years) group who are unable to work due to their illnesses. Thus, the loss of productive resources due to non– working of TB patients further weakens the economy. The cost involved towards treatment and care of sick individuals also adds to the burden on the economy of the families of the patients [1-3]. Eventually, a high financial (about trillion of US dollars) burden on global economy and existing global TB scenario alarm us about the difficulties to be faced in meeting the challenge of global TB control. All this has compelled the health managers and scientific community to evolve some cost effective preventing and treatment strategies for global control of TB.
The history indicates that vaccines have proved to be most costeffective tools towards reducing and controlling several infectious diseases in human beings [4]. Likewise, it is expected and agreed that having anti TB vaccine might play a key role in controlling and thereafter in eliminating and probably, in eradicating TB from the world. By a successful anti TB vaccine the burden of treatment cost could be reduced appreciably [1-3]. Presently, an attenuated strain of Mycobacterium bovis called Bacille Calmette-Guérin (BCG), discovered almost a century ago, is the only approved vaccine available against tuberculosis [5]. However, its protective efficiency is not adequate and is arguable. Hence, there is an urgent need of a better vaccine and/or vaccination approach against TB. Over the past 15 years, intense efforts have been made in this direction and diverse promising candidate vaccines (which are at various stages of their evaluations), designed following various strategies, have been reported [1,2,4,6]. Nonetheless, no sufficiently effective vaccine has emerged as yet. Still more innovative approaches are required for developing more effective vaccine(s) or to improve the performance of existing vaccines/candidate vaccines. Here, a suggestion has been extended to improve the protective efficacy of anti TB vaccine(s) at the level of autophagy in professional antigen presenting cells (APCs) i.e. monocytes/macrophages and dendritic cells.
After exposure to MTB bacilli, about 90-95% of the healthy persons do not develop active TB rather transmitted MTB are either eliminated from the invaded tissues after their killing by the innate immune cells or they persist in the host under the containment of immunity leading to development of asymptomatic latent tuberculosis infection (LTBI). The remaining (5-10%) of the infected healthy subjects develop active disease indicating lack of immunity, in such individuals, to protect against MTB. Moreover, about 5-10% of the individuals having LTBI have the risk of converting into active TB during life time [7,8]. It is the active TB patients who act as a primary source of transmission of MTB to other individuals. Thus anti TB vaccine should be aimed to prevent developing of active TB after MTB infection and thereby in preventing transmission of TB from such individuals. This in turn would contribute marvellously towards global control of TB.
In principle, a vaccine is intended to create long lasting protective immunity through generation of memory cells against the antigens of the target pathogen [9]. In case of an effective vaccine, post vaccination infection with the respective pathogen would lead to generation of prompt and intense long lasting immune response (by stimulating the vaccine generated memory cells) which eventually would result in production of effector cells and bio molecules needed for their involvement towards protecting the host.
It is well established that protection against MTB is caused, primarily, by cell mediated immunity. Based upon studies with patients and experimental models, CD4+ Th1 (T-bet+; a master transcription factor for differentiation of naive CD4+ T to CD4+ Th1) mediated cellular immunity against MTB is considered to be crucial to provide protection [10-12] against developing TB. Further, it is known that using candidate vaccines against TB it could be possible to generate the memory cells to provide future protection against MTB infection [13,14]. However, as evidenced [15-18] in TB prone individuals, APCs are incapable of killing the invading MTB bacilli. As a result thereof, there may be no/insufficient processing and presentation of MTB derived antigens to re-stimulate vaccine generated memory CD4+ Th1 cells for their further involvement in inducing protective immunity. Hence, it is worth arguing that probably for such persons an anti-TB vaccine may not be successful in protecting the host [19].
Regarding CD4+ Th17 cells (RORγt; a master transcription factor for differentiation of naive CD4+ T to CD4+ Th17) and immunity against MTB, there are several evidences [20] describing involvement of CD4+ Th17 cells in providing protection against MTB infection. However, understanding on this aspect is still at experimental level, incomplete and evolving.
Autophagy [21,22] is an essential biological phenomenon which exists in all living eukaryotic cells and is involved in degradation of old, supernumerary, dysfunctional, unwanted intracellular macromolecular components, organelles and intracellular microbes prevailing in cell. The degraded products in turn may act as alternate metabolic substrates for de-novo biosynthesis of new bio-molecules and generation of energy in the host. Autophagy is also considered as an integral part of immunity through playing a role at the levels of innate as well as adaptive immunity. Normally, autophagy always occurs, at low basal levels, to maintain metabolic homeostasis in the cells. However, under stressful situations this process may be up-regulated whereby the occurrence of diseases like, diabetes, neurodegeneration and myopathies etc. is prevented. The three better understood pathways for autophagy are: (i) chaperone mediated autophagy: where translocation of cytosolic soluble proteins, having particular pentapeptide motif (KFERQ), into lysosomes is promoted by chaperones; (ii) microautophagy: where lysosomal membrane directly internalise the cytoplasm material by invagination (iii) macroautophagy: during this process, the cytoplasmic constituents to be eliminated (cargo) are sequestered inside the cell by making an envelope of membraned vesicles (called phagophores) around those. The covered structure thus produced, known as autophagosome, is then directed to fuse with lysosomes for degradation of unwanted intracellular components and thereafter their recycling inside the cells or removal from the cells. Among the three types of autophagies, macroautophagy (in rest of the text this would be represented by autophagy) is a predominant and most commonly studied form.
After entering the host, MTB bacilli are preferentially phagocytosed by APCs i.e. monocytes/macrophages and dendritic cells [15,23]. During phagocytosis, MTB bacilli are encapsulated in a membranous layer derived from cell wall of phagocytes. This enveloped structure, containing MTB inside it, is known as phagosome. Normally, internalized MTB bacilli are supposed to be killed and eliminated by invaded APCs after passing through steps like: acidification, maturation and fusion of MTB loaded phagosome with lysosome. However, in susceptible host, bacteria reside alive in phagosome, by withstanding (by employing various evading strategies to counter the antimicrobial mechanisms) the anti- microbial environment inside the APCs [15,23- 25]. In such individuals, MTB can successfully grow inside the APCs leading to development of disease.
Among the various antimicrobial processes, autophagy is considered to be highly effective bactericidal system [24]. On stimulation of autophagy, MTB loaded phagosome is enlayered by phagophores and thereafter the enveloped phagosome fuses with lysosome. Next, MTB bacilli present in fused phago-lysosome undergo degradative killing by lysosomal contents in the cell. Additionally, after degradation of MTB into fragments (including immunogenic peptides), autophagy helps in presentation (in combination with major histocompatiblity complex) of MTB derived antigen to naive CD4+ and CD8+ T cells [26]. Regarding CD4+ T mediated cell meditated immunity (CMI), IL-12 secreted by activated APCs polarizes the stimulated naive CD4+ T cells to effector Th1 cells which in turn take part in generation of CMI. With this phenomenon, a portion of effector CD4+ Th1 cells gets transformed to memory cells. During CMI the cytokines like: Tumor Necrosis Factor-α (TNF-α) and Interferon Gamma (IFN-γ) are produced which in turn trigger antimicrobial machinery existing in APCs [10-12] and thereby protect the host by killing of invading MTB.
It is considered that in case of healthy subjects who are prone to develop TB, vaccine may not be effective in protecting the host which could all be due to non-killing of the infecting MTB and thereby no/ insufficient presentation of MTB antigens for stimulation of vaccine generated memory CD4+ cells [15-19]. In line with this, there are experimental evidences indicating that stimulation of autophagy can help in destruction of invading MTB bacteria [26-27] and in enhancing the efficacy of BCG as a vaccine against TB [28]. Jagannath et al. [29-30] have shown that (i) Over-expression of antigens (Ag85B in their study) in BCG could enhance sorting of such antigens to auto-phagosome for enhanced antigen presentation and stimulation of CD4+ Th1 cell mediated immune response. It is thought to be due to formation of insoluble aggregates, of over-expressed proteins, in the cytosol of APCs, where antigens in particulate forms induce autophagy and are prone to be processed for presentation with MHC-II. (ii) Administration of rapamycin, could enhance efficacy of BCG by improving antigen presentation through increased expression of MHC-II molecules and due to induction of autophagy by repressing the constitutive autophagy regulating (suppressive) protein known as mammalian Target Of Rapamycin (m TOR). Moreover, IL-1β produced (by non conventional mode) due to induction of autophagy could also enhance CD4+ T cell mediated immune responses against MTB through facilitating the expansion of CD4+ T cells. Nevertheless, enhancing of autophagy by repeated administration of rapamycin (at low doses) along with BCG may be undesirable, as repeated administration of BCG is known to cause immune pathological complications [31].

Figure 1: Cartoon depicting various steps involving towards stimulation of memory generated CD4+ Th1 cells through induction of autophagy in antigen presenting cells (APCs)
Keeping all this information in view, it is worth hypothesizing that, possibly, efficacy of anti tuberculosis vaccine could be improved (at the level of CD4+ Th1 mediated CMI) involving autophagy. In endemic countries there would be high frequency of exposure of the individuals, living there, to MTB which may thus infect them again and again. Considering the available information on the role of autophagy in defending the host [24,26,27], it is tempting to hypothesize that frequent (at some intervals of time) triggering of autophagy in the APCs of anti TB vaccinated subjects, residing in TB endemic regions, might maintain up- regulated MTB killing capacity in APCs. Due to induced autophagy, the antigens derived from killed bacteria would be processed and presented in combination with MHC-II, by MTB invaded APCs to stimulate vaccine generated memory CD4+ Th1 cells. Moreover, secretion of IL-1β due to induction of autophagy [32] can also help in facilitating further expansion of antigen stimulated CD4+ Th1 and thereby in enhancing cell mediated immune response [33].
Also, IFN-γ produced by stimulated CD4+ Th1 cells may, in turn, activate the phagosomes inside the APCs for their fusion with lysosomes. Eventually, this may lead to killing of engulfed MTB bacteria present in phagosomes. Furthermore, IFN-γ produced during this phenomenon may activate the autophagy in APCs and thereby in killing of phagocytosed MTB bacteria [34]. Thus, IFN-γ activated autophagy could help in further production of IL- 1β which again could help in enhancing the CMI. In this way, a feedback amplification loop is generated between autophagy and CMI. It is possible that excessive accumulation of IL-1β and IFN-γ may have some immuno-pathological complications due to their pro-inflammatory behaviors. Hence, frequent elicitation (at some time intervals but in a controlled manner in terms of frequencies and dose of inducer to make the approach safe with minimum side effects) of autophagy could stimulate repeatedly the vaccine generated memory cells (Figure1) which otherwise may remain un-stimulated due to non killing of infecting MTB bacteria and thereby due to no/insufficient presentation of MTB derived antigen to stimulate CMI [19]. Employing this approach, the vaccine generated immune response might be kept sustained and elevated for protection of the vaccinated host against future invasion by MTB. This way the protective efficacy of anti TB vaccine (s) might be maintained.
Experimentally, it has been shown that infection of macrophages (employing U937, a human macrophage cell line) with Human Immunodeficiency Virus (HIV) can subvert autophagy [35] and thereby may make the intracellular system conducive for its replication as well as for invading MTB. However, some promising reports have poured in [36,37] describing induction of autophagy in macrophages to kill both HIV as well as MTB. Hence, manipulation at the level of autophagy appears to hold great potential for developing anti TB vaccines to protect host against TB even in situation of dual HIV- MTB infections.
Explorative studies on approaches (including physiological, biochemical, microbiological and pharmacological etc) to understand their immunestimulating (through triggering of autophagy) activities and optimal time intervals to trigger the autophagy to enhance the efficacy of anti TB vaccine are highly needed. Finding a worthwhile approach free from side effects or with non-significant side effects might help, significantly, in bettering the protective efficacy of anti TB vaccine(s) and thereby in global control of TB through prevention of MTB transmission. Apart, such an effective approach might act synergistically along with chemotherapy towards treatment of subjects having active or latent form of TB.
Regarding leprosy, no efficient vaccine is available as of now. Mycobacterium leprae, the causative agent of leprosy is also an obligate intracellular mycobacterium which again invades APCs and grows in them. In case the above suggested approach for improving the performance of anti TB vaccine proves to be successful for tuberculosis, then it would beworthwhile extending that for developing and improving anti leprosy candidate vaccine(s) as well.
Thanks to Juraj Ivanyi, Department of Oral Medicine & Pathology, Guy’s Campus, Medical and Dental Schools of Kings College, London, United Kingdom, for his constructive suggestions. Thanks to Infolep, Amsterdam, The Netherlands, for providing the scientific literature. Thanks to Indian Council of Medical Research and NJIL & OMD, Agra, for routine facilities.
There is no conflict of interest regarding this manuscript.
Download Provisional PDF Here
Article Type: Mini Review
Citation: Om Parkash (2015) Anti Tuberculosis Vaccine and Autophagy: A Possible Platform to Improve the Performance of Vaccine. J Infect Pulm Dis 1(2): doi http://dx.doi.org/10.16966/2470-3176.106
Copyright:© 2015 Om Parkash. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Publication history:
All Sci Forschen Journals are Open Access